Figure 1: Nasal vs Oral route for Contraceptives

Ambikanandan Misra*
1Faculty of Pharmacy, The Maharaja Sayajirao University of Baroda, Kalabhavan, Vadodara, Gujarat, India*Corresponding author: Ambikanandan Misra, Faculty of Pharmacy, The Maharaja Sayajirao University of Baroda, Kalabhavan, Vadodara - 390001, Gujarat, India, E-mail: misraan@hotmail.com
Family planning has been a topic of global advocacy mostly due to the health and economic benefits to individual and society. Extensive research is undertaken worldwide for the development of improved versions of existing methods as well as the development of new technologies for family planning. However, family planning still has remained as an unmet need especially in developing countries. According to a report published by Guttmacher Institute, one in seven married women in developing countries has perceived contraception as unmet need [1]. In spite of public awareness about family planning alternative among women, there have been some obstacles which are limiting the success of family of planning, which include: unavailability of contraception as a ready measure, the women’s concern for health risk and side effects of contraceptive methods, or they find contraception inconvenient to use. Pharmaceutical development in contraceptive delivery can surely help in overcoming these obstacles.
Oral contraceptives were first to benefit the health and family planning and reproductive lives since 1960s. They were based on combined use of estrogen/progestogen pills and progesterone-only pills which work by interfering with female reproductive cycle. Later, emergency contraceptive (EC) have evolved to prevent pregnancy after contraceptive failure or unprotected intercourse. EC pills can avoid pregnancy when taken up to 5 days after unprotected sex. Ethinyl estradiol and levonorgestrel are used together as EC which prevent ovulation (the release of an egg from an ovary), disrupt fertilization (joining of the egg and sperm), and inhibit implantation (attachment of a fertilized egg to the uterus).
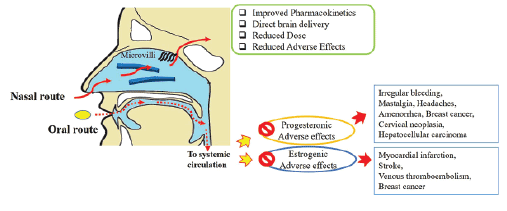
Oral contraceptive have condemned for long because of their estrogenic adverse effects such as myocardial infarction, stroke, venous thrombo embolism, breast cancer etc [2]. However, progesterones may also lead to progesteronic adverse effects like irregular bleeding, mastalgia, headaches, amenorrhea, breast cancer, cervical neoplasia, and rarely hepato cellular carcinoma [3]. Besides side effects, several drug interactions to anticoagulants, antibiotics, or anticonvulsant drugs are at risk due to hepatic enzyme induction [4]. In a long-term course of treatment they may interfere with hepatic protein synthesis of procoagulatory and fibrinolytic proteins and also lead to fatty liver [5].Therefore, there is a need of pharmaceutical development in female contraceptives to improve the safety and user compliance (Figure 1).

Figure 1: Nasal vs Oral route for Contraceptives
Oral route of contraceptives delivery suffers from another major drawback due to low bioavailability. Though 17α-ethinyl estradiol was developed as orally active oestrogen, was later reported to undergo significant metabolism in several animal species. The presence of hydroxyl groups at 17 position makes it susceptible to sulphate and glucuronide conjugation. Hirai et al reported that 40% gets metabolized in gut wall and 79% in liver through first-pass metabolism [6]. Similarly, Norethisterone (NET), progesterone, has been reported to have absolute bioavailability of about 60% in women [7]. Therefore, low bioavailability and high side effects demand that the alternative to classic oral and injectable routes be sought for administration of contraceptive.
The nasal route provides a useful way of administering a range of systemic drugs. The nose, due to presence of microvilli over epithelial surface offers large surface area for drug absorption and highly vascularized sub-epithelial layer helps to directly pass to systemic circulation and overcome first-pass metabolism, thereby reducing dose and accompanied side effects, a quick pharmacological action and direct brain delivery as well [8]. Lipophilic drugs are absorbed more effectively and completely through nasal route [9,10]. The poor absorption resulting from mucocilliary clearance can be overcome by using mucoadhesive agents such as polycarbophil, chitosan etc.
The benefits in terms of bioavailability after administration of contraceptive steroids, 17β- ethinyl estradiol, 17α-ethinyl estradiol, through nasal route has been reported [11-13]. A preliminary clinical trial has been reported to study the effects of intranasal administration of NET in form of spray. Intranasal administration of NET for 20 days (100 µg/ day) of the menstrual cycle brought about reproductive-endocrine effects similar to daily administration of 300 µg of NET mini pill throughout the cycle [14]. The intranasal route reduced the overall contraceptive drug load on the body through increase in bioavailability and favorable endocrine effects. NET inhibited ovulation in one cycle. The estradiol-induced mid-cycle rise in Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH) was either suppressed or inhibited. Progesterone levels were indicative of luteal inadequacy. Results concluded that nasal sprays of NET can be well accepted with no adverse clinical effects or menstrual disturbances, thereby suggesting that intranasal administration of NET may be a viable method of contraception [15].
Another study has reported the effects of intranasal administration of NET on reproductive function in women with particular reference to its effects on folliculogenesis, cervical mucus, vaginal cytology, endocrine profile of gonadal and pituitary hormones, and endometrial morphology. The study included 8 volunteers (Women with age 28 to 39 years, weights 46 to 54 kg). The study concluded indications of anti-fertility effects after intranasal administration of NET. These effects were attributed due to effects on endocrine profile, endometrial morphology, and alterations in cervical mucus and vaginal cytology. The authors stated that, since steroid hormones are rapidly absorbed through the nasal mucosa, it can be used for delivery of rapidly metabolizing natural hormones to overcome the first-pass effect in liver and at the same time reducing side effects due to drug load on body without lowering contraceptive efficacy [16].
With the advent of nanotechnology several carrier based delivery systems such as liposomes, micro emulsions have been used to deliver emergency contraceptive [5,17]. They can offer advantages such as improved stability, sustained release, targeting, improved pharmacokinetics etc. Further, there have been several patents filed to utilize the nasal route for delivery of steroids [13,18,19]. Emergency contraceptives for nasal and/or pulmonary administration comprising of levonorgestrel optionally in combination with ethylestradiol have been disclosed. The draft comparison showed the relative efficacy of administration of solution, suspension and carrier based systems selected from micro emulsion and liposomes for orally used contraceptive agents namely levonorgestrel and ethylestradiol alone or in combination delivered by nasal route. The delivery of contraceptives via nasal route led to enhanced brain uptake and alleviation of side effects of oral contraceptives as evidenced from the pharmacokinetic studies in rats [13]. Liposomal formulation of levonorgestrel administered via nasal route showed greatly facilitated nasal absorption in rat, thus providing for rapid onset of contraceptive action when compared to the drug formulation in suspension form administered by oral route. However, the relative bioavailability for various formulation administered via nasal route was only found to be around 30% for drug solution and liposomal formulation due to mucocilliary clearance. The use of mucoadhesive polymers like chitosan, carbopol etc., further augmented the pharmacokinetics by improving bioavailability to more than 95% with significant enhancement in the plasma half-life more than 7 times compared to drug solution, thus accounting for reduced dosing frequency of 2 days as opposed to the daily administration without corresponding dose adjustments [17]. A similar study to check the efficacy of formulation for contraceptive effect using leuprolide acetate liposomal formulation containing chitosan as mucoadhesive polymer administered intra-nasally and leuprolide acetate solution administered subcutaneously. Azoospermia was observed in male rats 26 days post initiation of medication as also cessation of estrous cycle in female rats was observed. Moreover, the contraceptive effect observed was reversible [20].
The current development in the field of emergency contraceptives is meagre with less number of novel therapeutics available for meeting the increased needs of population of having ease of administration/ application and at the same time devoid of side effects, so as to improve patient compliance. Since most of the therapeutics employed in emergency contraceptive are hormones that are prone to a wide range of physiological barriers post oral administration requiring frequent administration doses and thus increasing the chances of side effects. There is a need for designing delivery system offering ease of administration and at the same time be economical and improves compliance for the intended purpose of achieving contraceptive effect. The nasal route can be regarded as a convenient and efficacious alternative to the currently employed administration routes for availing therapeutics benefits of contraceptive agents. However, currently there is a limited data to substantiate the clinical usability of nasal route for delivery of contraceptive drugs. This requires that randomized controlled clinical trials of sufficient size be taken to statistically prove the efficacy of nasal route for emergency contraception.
Download Provisional PDF Here
Article Type: Short communication
Citation: Misra A (2016) Nasal Route for Delivery of Emergency Contraceptives. J Pharm Anal Insights 1(2): doi http://dx.doi.org/10.16966/2471-8122.105
Copyright: © 2016 Misra A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access