
Table 1: Nutrient content of the experimental diets

Jorge Guevara*1 Nelson Tapia1 Cesario Condorhuamán1 Patricia Díaz1 Fernando Carcelén2 Sandra Bezada2 Clemer Abad3
1Agroindustrial Engineering, National University Mayor of San Marcos, Lima, Peru*Corresponding author: Jorge Guevara, Agroindustrial Engineering, National University Mayor of San Marcos, Lima, Peru, E-mail: guevaravet@hotmail.com
Producing inocua meat of guinea pigs supplemented with natural flora probiotic and commercial probiotic was the objective of this research work. 100 weaned male guinea pigs of 28 days of age, cieneguilla genotype were used. A completely randomized design with 4 treatments, 5 replications and 5 animals per replicate was used. The treatments were: T1: Control Diet (Without probiotics), T2: Control Diet + Natural flora probiotic, T3: Control Diet + Commercial probiotic and T4: Control Diet + Natural flora probiotic + Commercial Probiotic. Lasted 28 days. Feed intake was higher in guinea pigs fed of natural probiotic diet with 1330 g, followed by guinea pigs fed of the control diet without probiotic with 1309.1 g, then the guinea pigs with commercial probiotic diet with 1307.5 g and finally the lower consumption guinea pigs treatment natural probiotic + commercial probiotic with 1298.3 g, without statistical difference between treatments. The greater weight gain obtained guinea pigs of the control diet without probiotic with 493.0 g, followed by guinea pigs treatment with natural flora probiotic with 492.1 g, then the guinea pigs treatment with natural flora probiotic + commercial probiotic with 450.0 g and finally the lower weight recorded guinea pigs fed the diet with commercial probiotic with 432.7 g, showed statistically significant differences between treatments. Feed conversion was better in the guinea pigs of control treatment without probiotic and with natural flora probiotic treatment with 2.8 and 2.9 respectively, followed by guinea pigs fed with commercial probiotic and fed natural flora probiotic + commercial probiotic with 3.2 and 3.1 respectively, without statistical difference between treatments. The highest carcass yield showed the guinea pigs of commercial probiotic treatment with 69.7%, followed by of guinea pigs with natural flora probiotic treatment + commercial probiotic with 68.6%, after of guinea pigs control treatment without probiotic with 68.0% and the lowest carcass yield showed of guinea pigs natural flora probiotic treatment with 67.7%. Showed no statistical difference between treatments. Also no guinea pigs were recorded in the presence of diseases or deaths guinea pigs.
Guinea pigs; Inocua meat; Natural flora probiotic; Commercial probiotic
Guinea pig breeding is entering a new phase that is to become a food alternative not only in our country but outside it, in this perspective, exploitation represents a good investment opportunity; however poor driving conditions and existing systems guinea pig breeding biosecurity involves constant stress and poor resistance to disease.
The economic impact of diseases is very important because of its high incidence means veterinary treatment, demand for time and labor, delayed physical development and even increased mortality. Faced with this problem, the most common form of control problems is through the use of antibiotics which are incorporated through balanced food animals as growth promoter antibiotic (APC).
This situation has caused great concern worldwide due to development of resistance of pathogens and the transfer of this resistance to human pathogens. To reduce the use of antibiotics in animal production have been evaluated several natural alternatives in chickens and pigs as successful probiotics to strengthen the immune system and increase resistance to disease.
Probiotics are live microorganisms, whose mode of action includes not only changes the pH of the gastrointestinal contents, but a number of direct effects add up as: antagonistic to the colonization of enteropathogenic bacteria or competitive exclusion action [1,2], decreased pH, neutralize toxins, bactericidal activity and beneficial effect on the immune system [3,4]. Similarly, the availability of amino acids increase and improve the efficiency of energy use [5] and other dietary components such as fiber for use as an energy source [6].
The objective of this research was to produce safe meat supplemented with probiotic cuy natural and commercial probiotic flora replacement of antibiotic growth promoters
The research was conducted in Agroindustrial Engineering of San Marcos - Lima - Peru. The pools were built of brick with separations of plywood, with a dimension of 1.0 × 1.0 and 0.37 m. tall, with 5 guinea pigs were housed. 20 pools were built in total. A feeding trough clay and china clay coated by pond, with a capacity of 250 grams and 250 ml respectively was used.
The characteristics of feed used in this experimental work are seen in Table 1. The green alfalfa forage was 10% of the live weight, which one half was distributed in two parts in the morning and one in the afternoon. Drinking water is offered daily and this was clean and fresh, for it is washed drinkers.
The probiotic natural flora was obtained from previously isolated strains scraping the epithelium and intestinal sections content guinea pig (Cavia porcellus) neonates (1-7 days), which were previously identified by molecular techniques based on bioinformatic analysis and sequencing of the gene 16S rDNA [7].Strains identified at genus and species were evaluated for their probiotic capacity by the following tests described in the literature: Determination of lactic acid production, determination of production of organic acids, determination of citric acid, determination of bactericidal activity, determining bacteriostatic activity, resistance to antibiotics and resistance to gastric acidity (pH) and bile salts.

Table 1: Nutrient content of the experimental diets
The commercial probiotic Lactobacillus based was obtained from the market for food supplies. Was administered orally 1 ml per animal for a week according to each treatment, and then at 2 weeks was again orally administered 1.5 ml per animal.
100 male guinea pigs, weaned 28 days old, with an average weight of 320 g, race Peru, which were distributed according to completely randomized design (CRD) with 4 treatments and 5 repetitions were used. A replay represented by a group of five guinea pigs housed in a pool. The treatments were: T1: control diet (no probiotics), T2: control diet + probiotic natural flora, T3: control diet + commercial probiotic and T4: control diet + probiotic + probiotic natural flora of trade
Data were analyzed using the SAP program and for the comparison of averages Duncan’s test was used. Also for taste testing different scale test, ANOVA and Friedman test was used.
The parameters evaluated were:
It is determined weekly and accumulated and for that weekly consumption of feed and forage weighed; not to fall into errors feed waste was avoided, the residue weighed and the net consumption thereby obtained. The result of such calculations was dry matter.
It is determined weekly and accumulated, the animals were weighed individually at baseline and weekly, at the same time (08:00) before the food supply. The total weight gain was obtained from the difference between the final weight and the initial weight assessment. For this parameter the animal avoided the night before eating and not have an error in weight.
It was obtained from the relationship between food intake and dry matter weight gain and accrued weekly and this factor an indicator of the goodness of the food processing animal tissue.
It is determined at the end of the experiment, benefiting in total 20 animals (5 per treatment and randomized) subjected to 12-hour fast. The housing included skin, head, legs and Guts: heart, lungs, liver and kidneys.
The number of animals suffering from gastrointestinal diseases, dead guinea pigs and others are determined.
Table 2 shows the total dry matter intake weekly food guinea pigs. It is observed that feed intake was higher in guinea pigs fed the diet with natural probiotic with 1330 g, followed by the guinea pigs fed the control diet without probiotic with 1309.1 g, then the guinea pigs diet with commercial probiotic with 1307.5 g and finally the lower consumption of guinea pigs treated with natural + commercial probiotic probiotic with 1298.3 g.
According to ANOVA, with a significance level of 0.05 we conclude that the sample evidence indicates that there is no significant difference for dry matter intake in the different treatments. The increase in dry matter intake increases from week to week because guinea pigs have higher requirements for growth, maintenance and fattening.
Higher than those reported by [8], although this author mentions that consumption of dry matter in guinea pigs with different levels of probiotics consumption does not increase results. Also [9], as research indicates that the probiotic sows not affect feed intake.
These results were lower than those reported by [10] online in Peru guinea pigs, probably due to the type of probiotics that the author used in the experiment being Lactobacillus + Yeast, different from those used in this research was natural probiotic flora.
Higher than those reported by [11], probably because these authors used Saccharomyces cerevisiae and Enterococcus faeciumk probiotic unlike this research where the probiotic natural flora or probiotic native was used in the treatment of guinea pigs.
The results overweight and gain weekly weight per treatment average seen in the Table 2, where it is seen that the guinea pigs who obtained greater weight at the end of the experiment were fed the control diet without probiotic with 903.4 g, followed by cuyes probiotic treatment with natural flora with 896.6 g, guinea pigs after treatment with natural probiotic + probiotic flora commercial 865.8 g lower weight finally recorded guinea pigs fed the diet with commercial probiotic with 851.9 g. This indicates that the natural flora probiotic gain more weight than with the commercial probiotic.
The greater weight gain obtained fed the control diet without probiotic with 493.0 g, followed by treatment with probiotic guinea pigs of natural flora with 492.1 g after treatment guinea pigs naturally probiotic + probiotic flora commercial 450.0 g guinea pigs finally recorded lower weight guinea pigs fed the diet with commercial probiotic with 432.7 g.
A 0.05 significance level there is significant difference in weight gain of guinea pigs according to the treatments provided. It is observed that weight gain between the guinea pigs of the control diet and the natural flora probiotic is only numerical difference, suggesting that the diet with natural flora probótico of similar weight gains are obtained commercial farms of guinea pigs with the difference that in this innocuous, antibiotic free meat is obtained.
[8] reported earnings similar to that found in this study in guinea pigs fed L. acidophilus, giving greater weight gain from the fifth week of weight assessment.
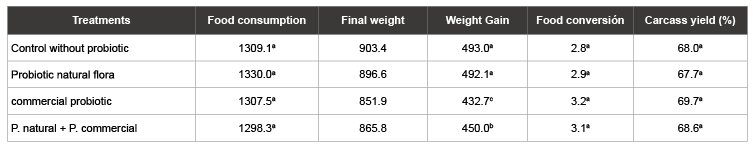
Table 2: Productive parameters of the guinea pig/animal/treatment (g)
a. Equal letters in columns indicate no statistical difference (P>0.05)
Similar to those reported by [12], who investigated in chickens weight gain recorded for chicks under treatment based probiotic Lactobacillus ssp and a control treatment without inclusion of probiotic. Weight gain for pullets with probiotic was 450 g, while the control treatment gained 415.93 g during the 42 days of investigation.
Similar to the results of [9] in piglets at birth, who gets greater weight gain in the control group (without probiotics), although in this study the difference was only numerical no health problems were reported guinea pigs fed with natural probiotic flora.
These results agree with those reported by [10], who published similar weights to those found in this study also are lower than the results reported by [11], probably because the probiotics used were different and the region where the research was conducted, as these authors saw and implemented in this paper on the coast.
Weekly results on feed conversion are shown in Table 2. It was found that feed conversion was better in control treatment guinea pigs and guinea pigs without probiotic treatment with natural probiotic flora 2.8 and 2.9 respectively, followed by the guinea pigs commercial probiotic fed and fed with natural probiotic + probiotic flora trade 3.2 and 3.1 respectively.
The ANOVA, we conclude to a 0.05 significance level that the sample evidence indicates that there is no statistically significant difference in feed conversion for different treatments; but if you can see a numerical difference between treatments.
The feed conversion results agree with those obtained by Tortuero (1993), who showed that the supply of pure strains of Lactobacillus acidophilus in broilers decreased malabsorption syndrome and produced an improvement in feed conversion. About the same indicates [8], the guinea pigs in the control group (without probiotic) was poorer than guinea pigs fed with probiotics.
Results higher than those found by [10] and reported by [11], probably because these authors used only probiotics trade and in the current investigation showed the best feed conversion of the guinea pigs treated with probiotics of natural flora or native.
In the Table 2 performance results as a percentage of housing and treatment are discussed. Higher yield of housing guinea pigs commercial probiotic treatment with 69.7%, followed by treatment with probiotic guinea pigs naturally + commercial probiotic flora 68.6%, then the guinea pigs control without probiotic treatment with 68.0% and the lowest was observed carcass yield guinea pigs showed treatment with probiotic natural flora with 67.7%.
The ANOVA concludes with a significance level of 0.05 that the sample evidence does not show statistically significant difference for the performance of housing in the different treatments.
These results agree with those reported by [8], who indicated that the carcass yield was higher for the supplemented probiotic treatment, although none of the variables evaluated in the different treatments were statistically different.
Similar results to those reported by [10] in guinea pigs supplemented with probiotic Lactobacillus + Yeast with an average of 66% return on housing. The lower the time reported by [11] who achieved average yield of 73% in housing guinea pigs supplemented with S. cerevisiae and E. faecium, unlike this investigation where the probiotic natural flora was used.
No guinea pigs illness or deaths were reported. Similar results to those reported by [10] in guinea pigs + supplemented with probiotics Lactobacillus and yeast reported by [11] in guinea pigs supplemented with S. cerevisiae and E. faecium, unlike the research which was used natural probiotic flora.
This is because ingested probiotics in sufficient quantities remain active in the intestine, contribute to the balance of the intestinal bacterial flora and boost the host immune system [4,13]. Natural probiotics and commercial plant increases the immune system of the gastric and intestinal mucosa, also are able to adhere to the intestinal mucosa and stimulate phagocytic cells more efficiently than other bacteria [14].
In the present research work it was reached the following conclusions:
With the inclusion of probiotics and probiotic natural commercial plant supplemented in the diet of guinea pig was able to produce safe meat.
Feed intake, feed conversion and carcass yield showed no statistically significant difference, the difference was only numerical, but weight gain was significant statistical difference, showing the biggest gains guinea pigs probiotic treatment with natural flora and treatment without probiotic.
Download Provisional PDF Here
Article Type: Research Article
Citation: Guevara J, Tapia N, Condorhuamán C, Díaz P, Carcelén F, et al. (2016) Production Inocua Meat Of Guinea Pigs Supplemented With Natural Flora Probiotic And Commercial Probiotic. J Epidemiol Public Health Rev 1(1): doi http://dx.doi. org/10.16966/2471-8122.103
Copyright: © 2016 Guevara J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access