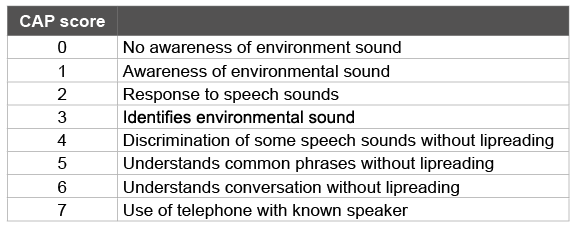
Table 1: Category of Auditory Performance (CAP)

VR Sinha1 Ruchika Mittal1* Ajith Nilkanthan1 Awadhesh Mishra1 DK Singh1 AV Ramesh2 Satish Nair3 Poonam Raj1
1Army Hospital (R&R) Delhi Cantt – 110010, India*Corresponding author: Dr. Ruchika Mittal, M-37C Rajouri Garden, New Delhi 110027, India, Tel: +919810961266; E-mail: ruchikamittal07@gmail.com
Objective: The results of cochlear implantation in patients that present with auditory neuropathy spectrum disorder (ANSD) patients have been variably reported. The effectiveness of the treatment modality varies in such patients. Hearing rehabilitation in this group of patients has been very challenging. Considerable controversy exists whether to provide conventional amplification (hearing aids or personal FM systems) or cochlear implants (CI) to children with ANSD. With this background, we present our experience with select subset of ANSD cases that derived benefit from cochlear implant.
Methodology: We studied and evaluated all the paediatric cases (i.e. less than 12 years of age) using audiological test battery. The cases with other co-morbidities, abnormality of auditory nerve and cochlea were excluded. Diagnosed cases of ANSD were given bilateral, behind the ear, digital hearing aids as per their behavioural responses for 6 months and enrolled for communication development in the auditory verbal habilitation program. They were evaluated for auditory perception using Category of Auditory Performance scoring system. The ANSD cases that derived “intermediate benefit” as per our criteria at 6 months post hearing aids were taken up for unilateral cochlear implant. All the implanted ANSD cases continued in auditory verbal habilitation program of our hospital and their progress on auditory perception post cochlear implant was monitored using CAP scoring system.
Results: A total of 1313 cases were evaluated for hearing loss. Out of these 65 cases were detected to have ANSD (42 bilateral and 23 unilateral ANSD).Unilateral ANSD cases were excluded from the study. Hearing aids were fitted in all the bilateral ANSD cases. After 6 months of hearing aid fitting thirteen ANSD cases showed “intermediate benefit” and were taken up for cochlear implant. After 6 months of implant usage all ANSD cases showed “good” progress on CAP.
Conclusion: Hearing aid trial should be given to all the cases diagnosed with ANSD and those who derive “intermediate benefit” from hearing aids and AVT should be considered for cochlear implant.
Auditory Neuropathy Spectrum Disorder (ANSD) is a pattern of hearing loss characterized by preservation of outer hair cell function despite absent brainstem auditory evoked responses. Intact outer hair cell function is demonstrated by the presence of Otoacoustic Emissions (OAEs) and / or measurable Cochlear Microphonics (CM) whereas no synchronous neural activity is seen on acoustically evoked auditory evoked brainstem response testing [1]. The underlying cause may be congenital or acquired. Congenital causes include genetic abnormalities, perinatal asphyxia, orhyperbilirubinaemia. Acquired causes include infections, demyelination disorders and vascular causes. Postulated sites of lesion in ANSD include selected outer hair cells, inner hair cells, the synapse between hair cells and the auditory nerve, neural dendrite/axon, myelin sheath, or spiral ganglion cells [2,3]. ANSD was initially believed to be very rare. However, various studies have shown that ANSD is much more frequent than previously believed, with its prevalence estimated to range from 0.5% to 15% [4] and incidence ranging from 2.4% to 14-15% in children with severe to profound sensori-neural hearing loss [1]. Therefore, dealing with hearing loss in ANSD is important. The effectiveness of the treatment modality varies on an individual basis. Hearing rehabilitation in this group of patients has been very challenging. Hearing aids, frequency modulation (FM) system and cochlear implantation are current treatment options for AN/AD, although the benefits of these interventions are variable and under much investigation [5].
Considerable controversy exists whether to provide conventional amplification (hearing aids or personal FM systems) to children with ANSD. About 50% of the children in one study gained significant benefit [6], although some clinics report much lower success rates [7]. Moreover, cochlear implants (CI) in ANSD are also a debatable issue. If the site of the lesion is the cochlea, then bypassing the inner hair cells with direct stimulation of the vestibulocochlear nerve should produce good results. However, if the pathological condition lies in the nerve itself as in ANSD, then electrical stimulation (cochlear implants) might be expected to be subject to the same limitations as acoustic stimulation (hearing aids) [8]. Thus it may be logically inferred that those ANSD cases that get some benefit from hearing aids would benefit better from cochlear implants, as it suggest that the nerve is at least partially functional.
With this background we planned to evaluate the hearing outcomes in those ANSD cases that derived some benefit from hearing aids and subsequently underwent implantation.
We studied all the paediatric cases (i.e. less than 12 years of age) referred to our tertiary care hospital’s outpatient department of ENT for hearing evaluation from March 2008 to March 2013. All the cases were evaluated using audiological test battery consisting of Behavioural Observation Audiometry (BOA) / Pure Tone Audiometry (PTA), Impedance Audiometry, Diagnostic Otoacoustic Emissions and click evoked Brain Stem Evoked Response Audiometry (BERA). BERA with both condensation and rarefaction polarity was performed in cases where wave V was absent at highest intensity levels to look for cochlear microphonics (CM). The CM were considered to be present if the response waveform showed a 180 degree phase shift with the change in stimulus polarity from rare fraction to condensation [9,10]. The authenticity of the CM response was confirmed through test turns in which the tube of insert phone was clamped to prevent the acoustic signal from reaching the ear canal. Under these circumstances, genuine cochlear potentials were abolished while the stimulus artifact remained unaltered [6]. All the audiological testing was performed in sound treated room and the uncooperative or young children were sedated with appropriate dosage of Syrup Triclofos oral solution BP (0.5 ml/kg) prior to objective testing. All the cases diagnosed with hearing loss were also referred for MRI brain, paediatric neurologist evaluation and clinical psychologist assessment and opinion to rule out any structural abnormality of cochlea, eighth nerve or other central nervous system (spell out: central nervous system first time it is used) (CNS) disorders.
The criteria used for audiological diagnosis of ANSD were evidence of normal or near normal cochlear hair cells (sensory) function (preservation of otoacoustic emissions and / or cochlear microphonics) absent or abnormal auditory nerve function (absent or severely abnormal auditory brainstem potentials) [5]. The etiological work up included full paediatric evaluation including imaging and genetic evaluation as indicated [2].
All the cases diagnosed with bilateral ANSD were given bilateral, behind the ear, digital hearing aids as per their behavioural responses for a minimum of 6 months. These cases were also enrolled for communication development in the auditory verbal habilitation program of our hospital. They were evaluated for auditory perception at the time of hearing aid fitting and after 6 months of hearing aid fitting using Category of Auditory Performance (CAP) scoring system [11]. The criterion used to evaluate the benefit post intervention is mentioned in Table 1.
On the basis of the CAP scores, benefit was classified into no benefit, intermediate benefit and good benefit (Table 2). ANSD cases that derived “intermediate benefit” at 6 months post hearing aids were considered for unilateral cochlear implant with straight electrode array. Cochlear implant was performed using standard techniques. Any complication during the surgery was noted and post operative X-ray Skull Modified Stenver’s view was taken to confirm the correct placement of electrodes. All the implanted ANSD cases continued in auditory verbal habilitation program of our hospital. Their progress on auditory perception post cochlear implant was monitored for a year at 6 and 12 months using CAP scoring system.
1313 cases were evaluated for hearing loss from March 2008 to March 2013 in our tertiary care hospital. Out of these 65 cases were detected to have ANSD (42 bilateral and 23 unilateral ANSD). The identified cause in 72% of ANSD cases was hyperbilirubinaemia and in 28% of the cases the cause could not be identified. Unilateral ANSD cases were excluded from the study.
Hearing aids were fitted in all the bilateral ANSD cases. At the time of hearing aid fitting and 6 months later the CAP scores were as depicted in Table 3. After 6 months of hearing aid fitting thirteen ANSD cases showed “intermediate benefit” (Table 4). The age and sex distribution of the ANSD cases depending upon the benefit derived from hearing aids is mentioned in table 5.

Table 1: Category of Auditory Performance (CAP)
Out of these thirteen ANSD cases that showed intermediate progress 54 % could detect environmental sounds, 38 % could respond to speech sounds and 8 % could identify environmental sounds.
These thirteen ANSD cases were taken up for cochlear implant using posterior tympanotomy with cochleostomy by various cochlear implant surgeons. All CI surgeries were uncomplicated and postoperative imaging confirmed the correct placement of the CI electrode array in all the cases. 9 cases were implanted with Medel Combo 40+, 3 with Advanced Bionics Hi Res 90K implant and 1 with Cochlear Nucleus Freedom straight implants.
After 6 and 12 months of cochlear implantation the progress of ANSD cases is depicted in table 6. After 6 months of implant usage all ANSD cases showed “good” progress on CAP (Table 5). 38% of the cases could understand common phrases without lip reading, 53% could discriminate speech sounds without lip reading and 8% could identify environmental sounds.
After one year of implant usage 77% of ANSD cases could understand conversation, 15% could understand common phrases without lip reading and 8% could do telephonic conversation as depicted in Tables 6 and 7.
The CAP scores were calculated at different time intervals of assessment and are presented in the form of mean ± standard deviation in table 8 for those ANSD cases that derived intermediate benefit from hearing aids and underwent cochlear implant surgery. Among those who underwent cochlear implantation, a statistically significant increase in the mean CAP score at the time of hearing aid fitting compared with the mean CAP score at 6 and 12 months post cochlear implant was observed.
The diagnosis of ANSD is a difficult task. The criteria used in the study to make diagnosis of ANSD are the most widely accepted criteria [6,9,10]. Buchmann et al. [12] reported that typical audiological findings of ANSD could also be present in cases with cochlear nerve structural deficiency. It was ruled out in our cases by normal radiological findings.
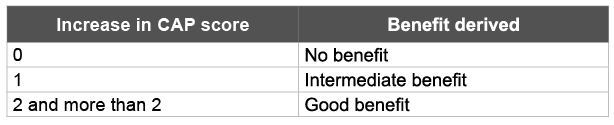
Table 2: Benefit derived Post intervention
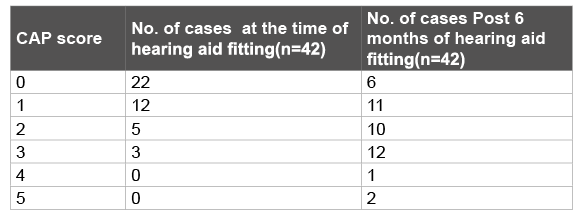
Table 3: CAP score Post hearing aid fitting at various intervals
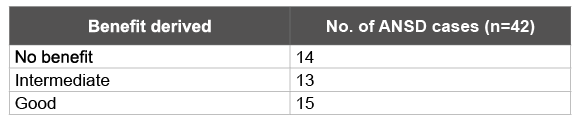
Table 4: Benefit derived after using hearing aids for 6 months
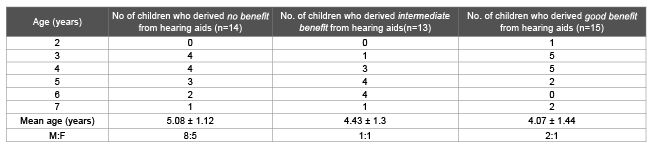
Table 5: Age and sex distribution of ANSD cases depending upon their benefit from hearing aids
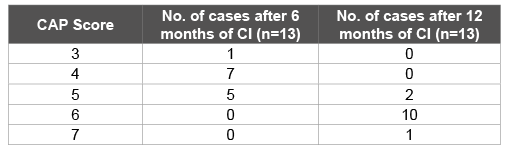
Table 6: CAP score at various intervals post Cochlear Implant (CI)
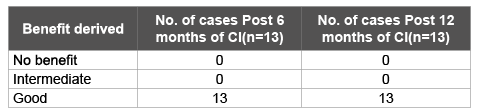
Table 7: Benefit derived from Cochlear Implant (CI) at various intervals
The progress of the cases was monitored using CAP. It is a rating scale that is rapidly applied and assesses the child’s auditory outcomes in everyday life. CAP is based on subjective assessment but has shown to have very high inter-user reliability [13]. Therefore, CAP is a well-established reliable post intervention outcome measure in hearing impaired children.
The revaluation was scheduled at 6 months post hearing aid fitting to decide whether to take up the child for cochlear implant as habilitation takes longer time and requires more coordination in ANSD cases.
We selected only those ANSD cases (31%) who showed “intermediate” benefit from hearing aids and excluded the ones who showed “no” (29%) and “good”(17%) benefit from hearing aids. The child’s age at the time of fitting for hearing aids had no relationship to whether the child derived any benefit from the aids. Benefit from the hearing aids is indirect evidence that the auditory nerve is at least partially functional. If acoustic stimulation is benefitting the child, then electrical stimulation would be beneficial too.
After 6 months of implant usage all ANSD cases showed “good progress” while these cases had shown only “intermediate progress” after 6 months of hearing aid usage earlier.
With six months of hearing aid usage 7/13 ANSD cases were aware of environmental sounds and 5/13 could respond to speech sounds and 1/13 could identify environmental sounds. However, with six months of implant usage 7/13 could discriminate some speech sounds without lipreading and 5/13 could understand common phrases without lip reading and 1/13 could identify environmental sounds. In one year 10/13 of the ANSD cases with implant usage could understand conversation without lip reading, 2/13 could understand common phrases without lip reading and 1/13 could even talk over telephone with familiar speakers.
In our study all the ANSD cases who received cochlear implant as per our criteria showed satisfying progress (Table 7). The Sydney Cochlear Implant Group centre has one of the largest cohorts of paediatric patients with auditory neuropathy undergoing cochlear implantation in the world (n=80). Many of the children proceeded to successful implantation, with a smaller number failing to gain significant benefit. They concluded that those who did not benefit may have dysfunction of the afferent neural synapse, cochlear nerve or beyond [14]. Therefore, evidence of some amount of auditory nerve sufficiency should be obtained prior to cochlear implant.
It is worth mentioning that ANSD describes functional disorders and not anatomical ones and should not be confused as a clinical entity with structural defect of auditory nerve. Evaluation of benefit from hearing aids gives indirect evidence that the auditory nerve is functioning. Therefore, instead of recommending cochlear implant for all children with ANSD, it is reasonable to recommend a hearing aid trial for a specified time period to evaluate the benefits from amplification and then depending upon the benefits from hearing aid, cochlear implant should be recommended.
Emerging data suggest that pre-implantation electrical stimulation testing may be useful in determining CI candidacy in some ANSD cases [15]. At the present time, pre- implantation electrical stimulation is not an established requirement for implantation and these invasive techniques are still at a research stage and further work is needed [5].
There is a considerable variability in the outcomes of ANSD children receiving cochlear implant. Hearing aid trial should be given to all the cases diagnosed with ANSD and those who derived “intermediate benefit” from hearing aids and AVT should be considered for cochlear implant.
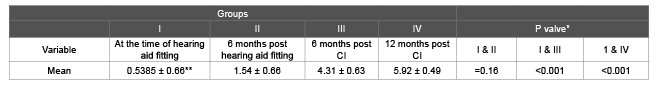
Table 8: CAP scores calculated at different time intervals with p valve for ANSD cases that underwent Cochlear Implant (CI) *p valve calculated using paired t Test, ** Baseline mean
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Sinha VR, Mittal R, Nilkanthan A, Mishra A, Singh DK, et al. (2015) Cochlear Implants and Auditory Neuropathy Spectrum Disorder. Pediatr Neonatal Nurs Open Access 1(2): doi http://dx.doi.org/10.16966/2470-0983.105
Copyright: © 2015 Sinha VR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access