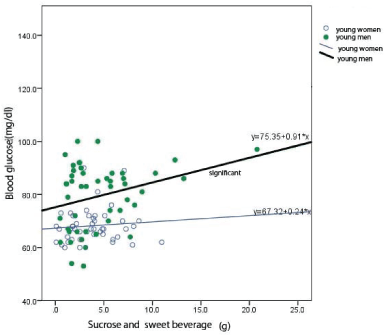
Figure 1: Relationship between the intakes of sucrose and sweet beverage with blood glucose levels in young people.


Shimizu F1 Ishii Y1 Ogawa M1 Takao T1 Matsuoka K2 Kato K2 Takada A3*
1Faculty of Human Life and Environmental Sciences, Showa Women’s University, Tokyo, Japan*Corresponding author: Takada A, International Projects on Food and Health (NPO), Sumidaku Ishiwara 1-30-6-802, Tokyo 130-0011, Japan, Tel: 81-338291849; Fax: 81338291847; E-mail: takadaa@mwd.biglobe.ne.jp
Background: Age and gender effects of various foods intakes on BMI (body mass index) or plasma levels of various factors such as glucose, insulin, and lipids have not been elucidated. We tried to examine these effects in Japanese young and old men and women.
Methods: Male (n=25 age; 60.8 ± 9.9) and female (n=39 age; 67.4 ± 7.5) acquaintances older than 50 years old and male (n=49 age; 20.7 ± 1.5) and female (n=47 age; 21.2 ± 0.7) college students were asked to participate in the experiments. BMI and various plasma factors were measured and correlations between these factors were calculated.
Results: Fasting blood glucose levels were higher in old men and women than in young men and women. There was no difference in plasma levels of insulin in young and old men and women. Old men and women took more energy, various kinds of foods such as proteins, lipids, and carbohydrates and polyunsaturated fatty acids than young men or women. There was a significant correlation between carbohydrate uptake and plasma levels of insulin only in young women. No significant correlations were observed between protein uptake and total amino acids, lipid uptake and total or LDL cholesterol, or carbohydrate uptake and blood glucose levels in young and old men or women. Plasma levels of triglycerides, total and LDL cholesterol, and remnant cholesterol were higher in old men and women than in young men and women. Plasma levels of poly unsaturated fatty acids such as EPA (eicosapentanoic acid), DHA (docosahexanoic acid), and arachidonic acid were higher in old men and women than young men and women. Blood glucose levels increased with the uptakes of sucrose and sweet beverage only in young men and plasma levels of insulin increased with the uptakes of cakes and confectionaries only in old men.
Conclusion: There were significant differences in plasma levels of various factors depending upon age and gender. Older people seem to have more factors related to atherosclerosis and obesity. There was no specific food related to increase in BMI in young and old men and women.
Glucose; Sucrose; Insulin; Lipid; Remnant; Cholesterol; Eicosapenanoic Acid (EPA); DHA Docosahexanoic acid (DHA); Body mass index (BMI)
Sex and gender are basic variables in scientific research in health and disease [1-3]. It is widely known that mortality rate increases with age and that women live longer than men.
Obesity is a risk factor of mortality [4], and influences of various food intakes on health and longevity have been documented well. There are some problems not clarified yet. For example, low carbohydrate high fat diets (LCHF) attract media attention, but some controversies exist about the outcomes. A range of dietary patterns promote health and has been shown to reduce risk of chronic disease [5]. Although some papers supported LCHF diets [6,7] dietary advices of the reduction of saturated fat, free sugars, and sodium and increased intake of whole grain cereals and fibers are recommended [8].
Systematic analyses commissioned by WHO have confirmed that free added sugars [9] and total fat intake [10] contributed to excess body fatness.
We have shown that there were no relationships between the intakes of sucrose and sweet beverage with BMI, fasting glucose levels or plasma levels of triglyceride [11,12].
Furthermore, we showed that GI (glycemic index) is very much different between old and young men even if the same foods with distinct structures are given, and insulin release to increase in glucose in young men is more sensitive than old men even if the same foods with distinct structures are given [13]. We also showed that GI was influenced by gender [11,13] and that various food intakes had nothing to do with BMI (body mass index) [12,14].
Although women and men have different physiology, expression of illness, and health-care outcome, women have not been studied in preclinical and clinical studies as much as men [1-3,15]. In fact, hypertension, acute myocardial, smoking, diabetes, and obesity account for 80% of risk of acutemyocardial infarction in both sexes, presence of diabetes is associated with 6-fold increase in women’s risk of coronary heart disease [15].
The National Institutes of Health (NIH) Revitalization Act, passed in 1993 and amended in 2001, mandates appropriate inclusion of women in all NIH-funded research [16]. In previous studies [11-14], we did not recruit old women for research because we were afraid that menopausal and general hormonal effects might affect results of effects of food intakes on health.
We now thought that it is important to include aged women in the present experiments to know more about effects of food intakes in old women. We now decided to include these women to analyze influences of age and gender on relationship between various food intakes on body mass index (BMI) and various plasma parameters. We did not include premenopausal women.
The objective of the study is to know differences in foods and energy intakes, various plasma parameters, and correlations between them among young and old men and women. As stated later, the exclusion factors were set so strict that these cohorts could be considered healthy. We especially paid attention to effects of the intakes of sucrose, sweet beverages, cakes and confectionaries on plasma levels of glucose and insulin.
This work has been approved by the Ethical committees of Showa Women’s University and NPO (non-profit organization) “International projects on food and health” and has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments.
We asked male and female acquaintances older than 50 years old and male and female college students to participate in the experiments. Acquaintances mean that these participants are personal friends of our group member. The sample sizes and ages of participants are as follows. Male (n=25 age; 60.8 ± 9.9) and female (n=39 age; 67.4 ± 7.5) acquaintances older than 50 years old and male (n=49 age; 20.7 ± 1.5) and female (n=47 age; 21.2 ± 0.7). Young males are students of Tokyo Institute of Technology and young females are students of Showa Women’s college. We did not ask premenopausal women to participate since data may be variable due to their hormonal influences so that sample sizes must be big to get statistically significant results. Dr. K. Matsuoka and K. Kato, who are internists, checked their health carefully and examined their blood samples then recruited them if there were no health problems such as diabetes, hypertension or not serious diseases experienced in the past. They did not smoke in the past. We also excluded people who took drugs for dyslipidemia, hyperglycemia, or hypertension. Doctors went to colleges to check health of participants. We collected blood samples early morning. Participants were asked not to eat anything after 21.00 PM the previous evening. Plasma specimens were collected for assays of blood parameters. We obtained an informed consent prior to conducting the protocol which had been approved by the Ethical Committee of Showa Women’s University and Saiseikai Shibuya Satellite Clinic.
Healthy participants were given self-administered diet history questionnaires and described answers on each item by recollection of diets they took (7 days dietary recall). We used a brief-type self-administered diet history questionnaire (BDHQ) by using which the Japanese Ministry of Health, Labour and Welfare reports national Nutrition Surveys. From these questionnaires, we calculated the intakes of energy, carbohydrate, fat, and protein.
Plasma factors were measured after plasma was separated from blood. Ethylenediaminetetraacetic acid (EDTA) was used as an anticoagulant. Blood glucose levels were measured by a hexokinase UV method. Insulin was measured by the CLEIA (chemiluminescent immunoassay) method. We also measured glycemic indexes after giving glucose and sucrose to participants, so that we did not use HbA1c as a marker of glycemia.
Lipid and lipoprotein concentrations such as total cholesterol, HDL (high density lipoprotein cholesterol), LDL (low density lipoprotein) cholesterol and TG (triglyceride) were determined using a Polychem Chemistry Analyzer (Polymedco Inc.). FFA (free fatty acid) and the concentrations of ω fatty acids such as arachidonic acid, DHA, and EPA were measured by a gas chromatography.
The thawed samples were deproteinized with acetonitrile followed by the amino acid analysis. Pre-column derivatization in the UF-Amino Station was automatically performed using an automated sample injector with the regent APDSTAG® (Wako Pure Chemical Industries, Ltd., Osaka Japan). Target free amino acids as derivatized compounds were separated under a reversed phase UHPLC condition and determined by the liquid chromatograph mass spectrometer.
Remnant lipoproteins (RLPs) were isolated from the serum by an immunoaffinity mixed gel containing anti-apolipoprotein A1 and antiapolipoprotein B100 monoclonal antibodies (Japan Immunoresearch Laboratories, Takasaki, Japan), and the cholesterol and triglyceride concentrations of the unbound fraction were measured as RLP cholesterol and RLP-triglyceride, respectively.
The results are presented as means ± SEM. Statistical significance of the differences between groups was calculated according by one-way ANOVA. When ANOVA indicated a significant difference (p<0.05), the mean values were compared using Tukey’s least significant difference test at p<0.05. Spearman’s correlation tests were used to examine statistical significance. We did not use Altman-Bland plots for the study because in the present studies there were little correlations between foods uptakes and plasma parameters, so we thought Spearman’s tests would be enough.
Results
Young men (n=49) and women (n=47) or old men (n=25) and women (n=39) were recruited for the experiments. (Table 1).
| a | b | c | d | statistical significances | |
| Young men (n=49) | Young women (n=47) | Old men (n=25) | Old women (n=39) | ||
| Age | 20.7 ± 1.5 |
21.2 ± 0.7 |
60.8 ± 9.9 |
67.4 ± 7.5 |
a vs.c: p<0.01 a vs.d: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 c vs.d: p<0.01 |
| Height (m) |
1.72 ± 0.06 |
1.58 ± 0.05 |
1.69 ± 0.07 |
1.57 ±.06 |
a vs.b: p<0.01 a vs.d: p<0.01 b vs.c: p<0.01 c vs.d: p<0.01 |
| Weight (kg) |
65.1 ± 8.9 |
51.4 ± 5.8 |
71.1 ± 13.1 |
50.6 ± 6.8 |
a vs.b: p<0.01 a vs.d: p<0.01 b vs.c: p<0.01 c vs.d: p<0.01 |
| BMI |
22.1 ± 3.1 |
20.4 ± 1.6 |
24.9 ± 3.7 |
20.5 ± 2.5 |
a vs.b: p<0.01 a vs.c: p<0.01 a vs.d: p<0.05 b vs.c: p<0.01 c vs.d: p<0.01 |
| Energy intake (kcal/day) |
1918 ± 554 |
1413 ± 407 |
2220 ± 544 |
1941 ± 535 |
a vs.b: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 |
| Protein intake (g/day) |
67.9 ± 23.6 |
51.8 ± 17.6 |
83.8 ± 27.4 |
80.0 ± 27.3 |
a vs.b: p<0.01 a vs.c: p<0.05 b vs.c: p<0.01 b vs.d: p<0.01 |
| Lipid intake (g/day) |
58.7 ± 22.7 |
45.3 ± 13.5 |
63.8 ± 19.8 |
60.9 ± 20.9 |
a vs.b: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 |
| Carbohydrate intake (g/day) |
264.2 ± 85.9 |
183.7 ± 61.8 |
268.0 ± 66.9 |
248.3 ± 76.9 |
a vs.b: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 |
Table 1: One -way ANOVA was used for evaluating statistical significance. a,b,c,d indicate values of young men and women or old men and women, respectively. p<0.05 was considered significant.
BMIs of young and old men were significantly higher than those of young and old women. BMI of old men was higher than that of young men. Energy, protein and lipid intakes of men were higher than those of women. Old men and women took more energy, protein, and lipid than young men and women, respectively. Fasting blood glucose levels were higher in old men and women than in young men and women. Fasting blood glucose levels were especially high in old women. There was not difference in plasma levels of insulin in young and old men and women.
Table 2 shows that old men and women took more energy, various kinds of foods such as proteins, lipids, and carbohydrates and polyunsaturated fatty acids than young men or women. Men took more energy, foods and fatty acids than women regardless of age. Young and old women took more carbohydrates than young and old men, respectively.
| Food intakes | a | b | c | d | ||
| Young men (n=49) | Young women (n=47) | Old men (n=24) | Old women (n=39) | Statistical significance |
||
| Energy | kcal |
1918 ± 554 |
1413 ± 407 |
2220 ± 544 |
1941 ± 535 |
a vs.b: p<0.01 b vs.c: p<0.01 b vs.d:p<0.01 |
| Protein | g | 67.9 ± 23.6 |
51.8 ± 17.6 |
83.8 ± 27.4 |
80.0 ± 27.3 |
a vs.b: p<0.01 a vs.c: p<0.05 b vs.c: p<0.01 b vs.d: p<0.01 |
| Animal protein | g | 39.5 ± 18.2 | 30.4 ± 14.4 | 49.2 ± 20.5 | 47.4 ± 19.8 | b vs.c: p<0.01 b vs.d: p<0.01 |
| Vegetable protein | g |
28.4 ± 9.1 |
21.4 ± 6.8 |
35.2 ± 9.3 |
32.6 ± 10.9 |
a vs.b: p<0.01 a vs.c: p<0.05 b vs.c: p<0.01 b vs.d: p<0.01 |
| Lipid | g | 58.7 ± 22.7 |
45.3 ± 13.5 |
63.8 ± 19.8 |
60.9 ± 20.9 |
a vs.b: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 |
| Animal lipid | g | 29.1 ± 14.5 |
21.2 ± 9.0 |
30.3 ± 13.0 |
29.0 ± 10.7 |
a vs.b: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 |
| Vegetable lipid | g |
29.7 ± 10.2 |
24.1 ± 7.3 |
33.6 ± 9.6 |
31.9 ± 11.9 |
a vs.b: p<0.05 b vs.c: p<0.01 b vs.d:p<0.01 |
| Saturated fatty acid | g | 16.8 ± 7.5 | 12.5 ± 4.5 | 16.5 ± 6.7 | 16.3 ± 5.6 | a vs.b: p<0.01 b vs.d: p<0.05 |
| Monounsaturated fatty acid | g | 21.3 ± 8.5 |
16.8 ± 5.4 |
23.0 ± 7.2 |
21.6 ± 7.7 |
a vs.b: p<0.05 b vs.c: p<0.01 b vs.d: p<0.05 |
| Polyunsaturated fatty acid | g | 13.6 ± 4.5 |
10.5 ± 3.2 |
15.9 ± 4.5 |
14.6 ± 5.3 |
a vs.b: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 |
| n-3 fatty acid | g | 2.5 ± 0.9 |
2.0 ± 0.8 |
3.4 ± 1.2 |
3.1 ± 1.4 |
a vs.c: p<0.01 a vs.d: p<0.05 b vs.c: p<0.01 b vs.d: p<0.01 |
| n-6 fatty acid | g | 11.1 ± 3.7 |
8.5 ± 2.5 |
12.5 ± 3.4 |
11.4 ± 4.0 |
a vs.b: p<0.01 b vs.c: p<0.05 b vs.d: p<0.05 |
| Cholesterol | mg | 358 ± 166 | 309 ± 141 | 461.1 ± 177 | 440 ± 188 | b vs.c: p<0.01 b vs.d: p<0.01 |
| Carbohydrate | g | 264.2 ± 85.9 |
183.7 ± 61.8 |
268.0 ± 66.9 |
248.3 ± 76.9 |
a vs.b: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 |
Table 2: Energy and foods intakes in young and old men and women.
There was a significant correlation between carbohydrate intake and plasma levels of insulin only in young women. No significant correlations existed between protein intake and total amino acids, lipid intake and total or LDL cholesterol, or carbohydrate intake and blood glucose levels in young and old men or women (Table 3).
| Young men (n=49) | Young women (n=47) | Old men( n=25) | Old women (n=39) | |
| Protein intake vs Total amino acids | -0.14 | 0.17 | -0.27 | |
| Lipid intake vs Total cholesterol | -0.14 | 0.18 | 0.16 | 0.24 |
| Lipid intake vs LDL cholesterol | -0.26 | 0.15 | 0.04 | 0.05 |
| Carbohydrate intake vs Blood glucose | -0.02 | -0.12 | 0.06 | 0.13 |
| Carbohydrate intake vs Plasma insulin | -0.13 | 0.45** | -0.08 | 0.09 |
Table 3: Correlations between foods intake and plasma parameters.
**p<0.01-Correlation coefficients and statistical significances were calculated by using Spearman’s correlation tests.
Plasma levels of triglycerides, total and LDL cholesterol, and remnant cholesterol were higher in old men and women than in young men and women. Triglyceride levels were higher in old men than in old women. The results may be related to lower incidences of cardiovascular diseases in women, since levels of triglyceride are one of risk factors of cardiovascular diseases. Higher levels of HDL-cholesterol in young and old women compared to those of young and old men may suggest that women have lower incidence of thrombosis. Plasma levels of HDL cholesterol were higher in women than men. Plasma levels of poly unsaturated fatty acids such as EPA (eicosapentanoic acid), DHA (docosahexanoic acid), and arachidonic acid were higher in old men and women than young men and women (Table 4).
| a | b | c | d | Statistical significances | |
| Young men (n=49) | Young women (n=47) | Old men (n=25) | Old women (n=39) | ||
| TG (mg/dl) | 75.7 ± 29.9 |
55.4 ± 29.0 |
124.6 ± 70.0 |
88.2 ± 54.8 |
a vs.c: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 c vs.d: p<0.01 |
| HDL-Cho (mg/dl) | 60.3 ± 11.4 |
71.3 ± 12.8 |
63.4 ± 14.3 |
75.3 ± 16.6 |
a vs.b: p<0.01 a vs.d: p<0.01 c vs.d: p<0.01 |
| LDL-Cho (mg/dl) |
104.9 ± 23.8 |
96.3 ± 23.6 |
128.4 ± 25.7 |
138.5 ± 33.4 |
a vs.c: p<0.01 a vs.d: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 |
| T-Cho (mg/dl) | 174.0 ± 24.0 |
177.1 ± 29.9 |
217.1 ± 28.0 |
232.6 ± 34.5 |
a vs.c: p<0.01 a vs.d: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 |
| RLP-Cho (mg/dl) | 2.7 ± 1.2 |
3.0 ± 1.5 |
6.2 ± 5.9 |
5.5 ± 3.2 |
a vs.c: p<0.01 a vs.d: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 |
| DHLA (μg/ml) | 33.4 ± 7.8 | 28.8 ± 8.3 | 39.2 ± 12.7 | 38.5 ± 12.6 | b vs.c: p<0.01 b vs.d: p<0.01 |
| AA (μg/ml) | 168.9 ± 37.1 |
172.1 ± 28.9 |
224.2 ± 53.6 |
215.6 ± 60.0 |
a vs.c: p<0.01 a vs.d: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 |
| EPA (μg/ml) | 28.2 ± 17.8 |
31.2 ± 19.5 |
88.7 ± 50.0 |
45.4 ± 29.1 |
a vs.c: p<0.01 a vs.d: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 c vs.d: p<0.01 |
| DHA (μg/ml) |
79.8 ± 22.6 |
83.0 ± 21.0 |
157.2 ± 50.9 |
136.0 ± 28.3 |
a vs.c: p<0.01 a vs.d: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 c vs.d: p<0.01 |
| EPA/AA | 0.17 ± 0.11 |
0.18 ± 0.11 |
0.41 ± 0.21 |
0.31 ± 0.14 |
a vs.c: p<0.01 a vs.d: p<0.01 b vs.c: p<0.01 b vs.d: p<0.01 c vs.d: p<0.01 |
Table 4: Plasma levels of lipids in young men and women or old men and women.
TG: Triglyceride; HDL-Cho: HDL-cholesterol; LDL-Cho: LDL-cholesterol; T-Cho: Total cholesterol; RLP-Cho: Remnant cholesterol; DHLA: Dihomo γ linoleinic acid; AA: Arachidonic acid; EPA: Eicosapentanoic acid; DHA: Docosahexanoic acid.
Blood glucose levels increased with the intakes of sucrose and sweet beverage only in young men and plasma levels of insulin increased with the intakes of cakes and confectionaries only in old men (Table 5) (Figure 1).

Figure 1: Relationship between the intakes of sucrose and sweet beverage with blood glucose levels in young people.
| Young men (n=49) | Young women (n=47) | Old men (n=25) | Old women (n=39) | |
| Sucrose and Sweet beverage vs Blood glucose | 0.29* | 0.09 | 0.10 | 0.04 |
| Cakes and Confectionaries vs Blood glucose | 0.01 | -0.11 | -0.38 | 0.08 |
| Sucrose and Sweet beverage vs Insulin | 0.27 | -0.10 | -0.08 | 0.00 |
| Cakes and Confectionaries vs Insulin | 0.23 | 0.13 | 0.42* | -0.17 |
Table 5: Correlations of intakes of sucrose and sweet beverage or cakes and confectionaries with blood glucose or insulin levels.
* p<0.05, Spearman’s correlation tests. Sweet beverage means the amounts (g) of sweeteners in the drinks.
The intakes of sucrose and sweet beverage resulted in increase in blood glucose levels in young men. The reexaminations of two young men whose glucose levels were lower than 60 mg/dl indicated that the data were in a range of normal fluctuation of glucose levels (Figure 2).
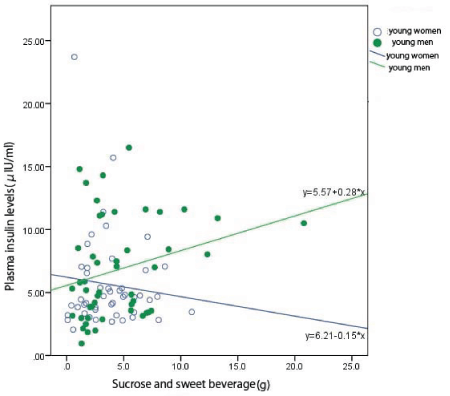
Figure 2: Relationship between the intakes of sucrose and sweet beverage with plasma insulin levels in young people.
The intakes of sucrose and sweet beverage did not affect plasma insulin levels in young people (Figure 3).

Figure 3: Relationship between the intakes of sucrose and sweet beverage with blood glucose levels in old people.
The intakes of sucrose and sweet beverage did not influence blood glucose levels in old people (Figure 4).
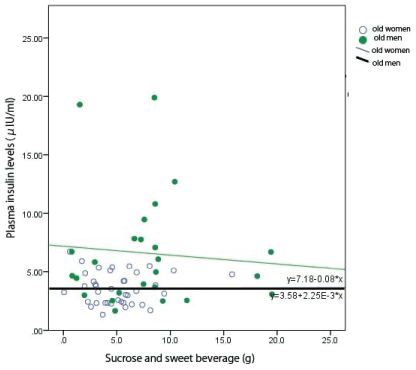
Figure 4: Relationship between the intakes of sucrose and sweet beverage with plasma levels of insulin in old people.
The intakes of sucrose and sweet beverage did not affect plasma levels of insulin in old people.
As stated in introduction, women and men are not only different in physiology but different in morbidity and mortality in cardiovascular, respiratory, musculoskeletal, immunological, gastrointestinal, neurological and renal diseases.
As to diseases related to obesity, the impact of obesity on the development of cardiovascular diseases seems to be greater in women than men as shown in Framingham Heart study [17]. In this study, the age-adjusted relative risk (RR) for new hypertension was highly associated with overweight status (men: RR, 1.46; women: RR, 1.75). New hypercholesterolemia and diabetes mellitus were less highly associated with excess adiposity. The age-adjusted RR for cardiovascular disease was increased among those who were overweight (men: 1.21 [1.05-1.40]; women: 1.20 [1.03-1.41]) and the obese (men: 1.46 [1.20-1.77]; women: 1.64 [1.37-1.98]).
In the present research we compared various foods intake, BMI, plasma levels of lipids and amino acids among healthy young men and women and old men and women.
Type 2 diabetes mellitus (T2DM) is considered to be closely related to obesity. There is a 3-fold excess fatal coronary artery diseases risk in women with T2DM compared with non-diabetic women [18]. Women with T2DM have higher adjusted hazard ratio of fatal coronary heart disease compared with men [19].
These data clearly show that there are gender differences in pathogenesis of diseases related to foods intakes.
Roles of various food intakes on health are complicated. One of the reasons may be that effects of various food intakes on body constituents may be different by age and gender.
Glycemic index (GI) is a simple factor proposed by Jenkins et al. in 1981 [20,21] which is defined as the area under the blood glucose curve measured two hours after consuming 50 g of test carbohydrates in relation to 50 g of glucose. We found that GI is influenced markedly by age and gender [12-14].
Rasmusen OW et al. [22] gave bread or rice to non-insulin depended diabetic patients and showed that there were no differences in insulinresponse areas between two groups and that GI of rice for males and females was similar. In Danish studies, GI was shown to be directly related to changes in total cholesterol in men but the association of GI and total cholesterol was shown to be inverse in women [23].
More surprising data were reported from Japan that the hazard rations of total stroke comparing the highest and the lowest quartiles of dietary GI were 0.78 in men and 2.09 in women. Such strong association was observed in women concerning the risk of ischemic stroke [24].
Using a meta-analysis, high dietary GL (glycemic load) and GI were shown to have significantly increased the risk of CHD in women but not in men [25].
Such data indicate that effects of foods intakes on health depend upon gender and women’s health seems to be more influenced by foods than men.
So it is important to examine female samples more precisely than before GI is influenced by many factors. Especially sensitivity to insulin contributes to GI values. We have shown that GI of glucose of old men is 153 when GI of glucose was set 100. Also GI of young women to sucrose was very low. These results may be due to high sensitivity to insulin and higher release of insulin in young men and women when carbohydrate was taken.
Recently, a net weight loss at 12 months from a 30 s active intervention by primary care physicians has been reported [26]. These results indicate that a precise knowledge and advice of obesity are important to reduce weight for obese people.
In regard to gender and age differences, metabolome studies were developed [27]. Total cholesterol in gender and age were identified as the principal factors explaining metabolome variability. Some metabolites were known to be higher in men than in women such as branched chain amino acids. Age-related changes in the concentrations of various metabolites have been observed [28].
In the present studies we examined differences of lipids, amino acids, blood glucose and insulin levels in young and old men and women. These parameters were used in relation to BMI so examinations were not as extensive as metabolome studies.
As shown in Table 3, plasma levels of triglycerides, LDL cholesterol or remnant cholesterol were higher in old people than in young people. We speculate impairments of lipid metabolism in older people. As well known, the prevalence of cardiovascular diseases is higher with age. When cross sectional studies that generated measurements of coronary atherosclerosis using state of art CT in individuals in Tsimane society in the Amazon region of Bolivia were performed atherosclerosis occurred when sedentary life style was absent [29].
Age seems to be the strongest risk factor among various risk factors measured and these observations suggest that atherosclerosis commences and progresses with age in healthy young adults. Our results confirm these contentions and plasma levels of factors related to atherosclerosis are shown to increase with age.
The present data indicate that intakes of proteins, lipids, and carbohydrate had nothing to do with plasma levels of total amino acids, lipids, or blood glucose levels in young and old men and women. Carbohydrate intakes resulted in increase in plasma levels of insulin only in young women.
As to intakes of sucrose, sweet beverage, cakes and confectionaries, we showed that BMI was not influenced [12]. Plasma levels of insulin increased after the intakes of sucrose and sweet beverage only in young men and plasma levels of blood glucose increased after the uptakes of cakes and confectionaries only in old men. Probably, insulin release did not increase upon increased intakes of sucrose in young men, and in old men insulin release increased upon intakes of cakes so that blood glucose levels did not increase.
Since plasma levels of insulin seem to be an indicator of obesity, the intakes of sucrose and sweet beverage may not result in increase in BMI in healthy young and old people [30].
As to the treatment of obesity, counseling by using individual and group session, low-calorie diet, physical activity such as aerobic activity, behavioral therapy such as daily monitoring of food intake and physical activity are recommended [31].
For achieving the effective weight loss, we must know basic parameters such as BMI, various food intakes, plasma levels of various parameters affecting body weight in healthy young and old men and women. The data presented in this article may be valuable to design new approaches to the prevention and treatment of obesity
Experiments were designed and performed by all of the authors. AT wrote the manuscript. Statistical analyses were done by TT. All authors read the manuscript and approved the final version. All the authors had responsibilities for the final content. No conflicts of interest for any author.
This study was supported by grants by NPO “International Projects on Food and Health.”
Download Provisional PDF Here
Article Type: Research Article
Citation: Shimizu F, Ishii Y, Ogawa M, Takao T, Matsuoka K, et al. (2017) Age and Gender Influence Differently on Various Foods Intakes, Body Mass Index (BMI), and Levels of Various Plasma Parameters in Young and Old Men and Women in Japan. Obes Open Access 3(3): doi http://dx.doi.org/10.16966/2380-5528.134
Copyright: © 2017 Takada A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access