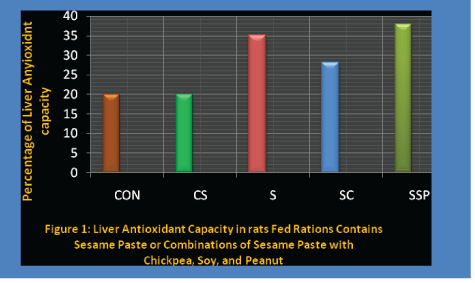
Figure 1: Liver antioxidant capacity in rats fed rations contains sesame paste or combinations of sesame paste with chickpea, soy and peanut.


Ghiath Sumainah1 Louay Laban2*
1Department of Biological Science, Syrian Private University, Damascus, Syria*Corresponding author: Dr. Louay Laban, Department of Nutrition, Al-Rasheed Private University, Damascus, Syria, Tel: +96894623170; E-Mail: drlouay@msn.com
Background: Consumption of sesame seed products is increasing worldwide, and sesame may be suitable as a quality protein source for preschool children when combined with other sources of plant proteins.
Sesame paste, or Tahini, is a traditional food in countries of the Middle-East, where other seed proteins such as the chickpea, soybean, and peanut are also locally available. Although the protein in sesame is low in lysine, but it’s rich in sulfur-containing amino acids in comparison to other seed proteins. Additionally, sesame contains potent water and fat soluble antioxidants with may affect immune function.
Materials & Methods: In this study, the growth response, liver antioxidant activity was measured. And in vivo cell-mediated immune function in Syrian hamster rats fed rations in which the protein content was 10% and was either all from sesame paste (S), one-fourth from sesame paste and three-fourths from chickpea (SC), or one-half from sesame paste and one-fourth each from soybean and peanut (SSP). There were 2 control rations, both containing casein as the protein source and fat from either soybean oil or sesame oil. Total fat content of the rations was similar. Syrian male hamsters were fed ad libitum for 4 weeks, after which they were administered a delayed-type-hypersensitivity (DTH) test of were assessed for liver antioxidant activity using αα-diphenyl-b-picrylhydrazyl.
Results: Protein utilization, as measured by the standardized PER (Protein Efficiency Ratio), was 2.50 for both control groups, 1.08 for group S, 1.59 for group SSP, and 2.18 for group SC. Liver tissue antioxidant activity was significantly higher in all 3 groups containing sesame paste vs the control groups. The DTH response was similar among the control groups and rate fed rations SC and SSP, but significantly greater in rats consuming the S ration. In summary, the SC formula containing protein that was 25% from sesame paste and 75% from chickpea is of good quality, supported normal cell-mediated immune function, and raised hepatic antioxidant level in comparison to the casein controls.
Conclusion: Sesame seeds products such as Tahini can play an important role as antioxidants especially for the hepatic functions.
Sesame; Tahini; Immune function; Chickpea; Protein utilization; Liver antioxidants
In various parts of the world efforts are being made to increase the consumption of vegetable protein from locally available sources. Sesame paste (Tahini) has a reputation as a popular food in East-Asia and in Middle-East countries [1]. It is made from ground, dehulled, dry-roasted sesame seed (Sesamunindicum L). The high oil content of approximately 60% consists mostly of oleic (39%) and linoleic (40%) acids [2]. Sesame paste also contains relatively high protein content (26%) and a low percentage of crude fiber and moisture [3].
The oil in sesame is remarkably stable and resistant to oxidative deterioration due to indigenous fat and non-fat antioxidants (tocopherols and lignans) [4-6]. Many of these antioxidants exist as the glucosides of sesamol, sesamin, sesaminol, sesamolinol, and pinoresinol [7]. There is evidence that the glucosides act as potent in vivo antioxidant substances [4,8]. In addition, they are capable of altering prostaglandin synthesis, thereby potentially affecting immune function [9]. Thus, increasing the rationary intake of sesame may improve health status. However, sesame use, especially for the preschool child, is hampered by the poor quality of its protein. Sesame is very low in lysine. Yet, the protein is exceptionally rich in sulfur-containing amino acids, whereas these building blocks are present at low levels in most other seed proteins [3]. Complementing sesame protein with other sources of seed protein has been shown to enhance overall protein quality [10,11]. Chickpea, peanut, and soybean are good complementary foods due to their widespread availability in the Middle-East and Asian countries.
Sesame seeds contain various compounds, including nonpolar, semipolar, and polar compounds. The different polarities of compounds in sesame seeds may render different antioxidant potentials [12].
Sesame seeds contain bioactive components including phenolics, vitamins, phytosterols, and polyunsaturated fatty acids, which provides a beneficial effect on human health [6]. Sesame seed lignans have been reported to have high antioxidant activity both in vitro and in vivo experiments [13].
Sesame seeds have potential antioxidant and anti inflammatory effects in various test systems, including humans, animals, and cultured cells through various pathways such as inhibition of COX, non enzymatic defense mechanism, inhibition of pro inflammatory cytokines, NF‐kB or mitogen‐activated protein kinase signaling, and prostaglandin synthesis pathway [14].
The purpose of our study was to evaluate sesame seeds in combination with chickpea, peanut, and soybean, as a means to significantly raise the protein quality and to possibly demonstrate its antioxidant and immune stimulating properties in the rat.
Sesame paste (Tahini), the standard commercial made from Sesamum indicum Linn, was purchased through a supplier in 20 kg plastic buckets from Lebanon (Al-Kanater Factory, Beirut). It was kept under refrigeration until used. A protein of the paste was partially defatted by repeated centrifugation (4000 × g) until no further oil would separate.
Raw chickpea (Cicero arietinurn Linn) was supplied from a commercial source, raw soybean seeds and heat-treated peanuts were obtained from the Department of Agronomy at the University of Damascus. The chickpea and soybean seeds were soaked with water (3:1) for 18 hours and then drained. Soaked soy was boiled in 0.5% Nabicarbonate for 45 minutes to inactivate the anti nutritional factors. Chickpea was autoclaved at 121°C for 45 min. Excess water was eliminated and the seeds were air-dried, freeze-dried, and then ground to pass 1 mm mesh. The proximate analysis is presented in table 1.
| Ingredients | Constituents as Percent | ||||
| Moisture | Protein1 | Fat | T-CHO2 | Ash | |
| Sesame paste, non- defatted | 2.81 | 20.60 | 57.10 | 16.68 | 2.81 |
| Sesame paste, partially defatted | 3.40 | 33.31 | 35.10 | 23.78 | 4.41 |
| Chickpea | 2.05 | 25.80 | 6.21 | 62.92 | 3.02 |
| Soybean | 2.02 | 38.82 | 20.01 | 35.93 | 3.22 |
| Peanut | 2.03 | 25.50 | 44.41 | 26.07 | 2.30 |
| Casein ANRC | 6.71 | 89.82 | 0.31 | 0.84 | 2.32 |
Table 1: Proximate analysis of the protein sources.
1Nitrogen to protein conversion factors are from USDA Handbook no.8 (Casein 6.38; Peanut 5.46; Soybean 5.11; Chickpea 6.25).
2Total carbohydrate calculated by difference between the sum of the weight of crude protein, total fat, moisture, ash, and the total weight of the food.
| Ingredients | Rations (%) | ||||
| Protein and Fat Sources | CON | CS | S | SC | SSP |
| Sesame paste, non-defatted | 8.4 | ||||
| Sesame paste, partially defatted | 30.0 | 14.5 | |||
| Chickpea | 33.8 | ||||
| Soybean | 10.4 | ||||
| Peanut | 5.8 | ||||
| Casein ANRC | 11.1 | 11.1 | |||
| Sesame oil (added) | 10.0 | 3.0 | 0.2 | ||
| Soybean oil | 10.0 | ||||
Table 2: Compositions of the rations.
*All rations contained 20% sucrose, 1.0% cellulose, 1.0% vitamin mix (AIN-93VX), 3.5% salt mix (AIN-93VX), 0.2% choline bitartrate, 0.002% TBHQ (antioxidant), and corn starch to 100%; the total nitrogen content and total fat content were approximately the same among the rations.
*CON = casein/soy oil; CS = casein/sesame oil; S = sesame paste; SC = sesame paste/chickpea; SSP = sesame paste (partially defatted) / soy flour/ peanut
All rations were formulated in the laboratory prior to the launch of the study and they were mixed in a Hobart mixer and fed in powdered from. There were two control rations, namely CON, made with casein with soy oil, and CS, containing casein and sesame oil. The groups were sesame paste-chickpea (SC) (protein source 25%: 75% respectively). Sesame paste alone (S) (protein source 100%), and sesame paste-peanut-soy (SSP) (protein source 50%:25%). All rations were formulated to give 10% total fat and 10% crude protein.
60 Syrian or golden Hamster Males (Mesocricetus auratus) which was available in the market at the time when the study was performed (weighing 41 ± 1 g) were purchased from the local laboratory animal breeding establishment (Damascus, Syria). These animals were acclimated to the laboratory for 3 days before initiating the experiment. All procedures for laboratory animals were in accordance with approval of the Institutional Animal Welfare Committee of the Syrian Private University. The Syrian hamsters were housed individually in stainless steel cages with 12-Hr light/dark cycle. They were randomly assigned into five groups (n=12), and given water and food ad libitum. The reason behind allowing Syrian Hamsters rats open amounts of food was an attempt to evaluate the palatability of different rations and to check their appetite. The leftovers were not recorded due to technical issues < their body weights and food intakes were recorded weekly for four weeks. Spilled food was collected and accounted for in tabulating weekly food intake.
PERs (Protein Efficiency Ratio) were calculated according to AOAC [12]. All values were then standardized to the CON group set to 2.50.
At the end of the PER (Protein Efficiency Ratio) assay 7 animals from each group were subjected to an in vivo Delayed-Type Hypersensitivity reaction (DTH) assay procedure as follows [15-18]. On day 1, the rats were anesthetized with Ketamine (80 mg/kg body weight), shaved over the abdomen, and painted with 100 μ of 1% DNFB (dinitroflourobenzene) in 4:1 acetone: olive oil. On the second day, another application of DNFB on the abdomen was made without anesthesia. On day 4, one ear was challenged with 50 μ of 0.5% DNFB in the vehicle. The other ear was painted with the vehicle to serve as the non - stimulated control ear. After 24 hours, ear thickness (swelling) was measured with a precision micrometer. The level of DTH response was quantified by the difference of ear swelling between the stimulated and non-stimulated ears in the same animal, expressed as the ratio of thickness of treated over untreated ear. A greater degree of swelling of the treated ear is indicative of a stronger immune response.
The remaining five Syrian hamster rats of each group were anesthetized. The liver was harvested, weighed and frozen pending analysis. Liver tissue was also collected and analyzed from the Syrian Hamster rats that were subjected to the DTH test above. Antioxidant activity was measured by the Glavind method using α, α- dipheylpicrylhydrazyl (DPPH) (Sigma Chemical, St. Louis, Mo) [18,19].
All data were subject to analysis of variance (ANOVA) using SAS, version 6.12 (SAS Institute, Cary, North Carolina). Duncan’s multiple range test was used to detect differences among treatment means if the F-value was significant. All effects were considered significant at P<0.05.
| Ration1 | Protein (g/100 g) | Food intake (g/28 d) | Body weight gain (g/28 d) |
PER2 |
| CON | 11.33 | 383ab ± 33 | 119a ± 6 | 2.50a ± 0.06 |
| CS | 10.63 | 369ab ± 7 | 107b ± 3 | 2.50a ± 0.05 |
| S | 10.69 | 235e ± 12 | 29c ± 1 | 1.08d ± 0.04 |
| SC | 10.38 | 373ab ± 10 | 92e ± 2 | 2.18b ± 0.05 |
| SSP | 11.56 | 347b ± 2 | 70d ± 2 | 1.59c ± 0.03 |
Table 3: Efficacy of Protein Utilization of rations containing sesame paste
or combinations of sesame paste with chickpea, soy, and peanut.
1CON = casein/soy oil; CS = casein/sesame oil; S = sesame paste; SC = sesame paste/chickpea; SSP = sesame paste (partially defatted) / soy flour/ peanut.
2PER’s were standardized to CON 2.50
3Mean ± SEM; Means with unlike superscript letters in the same column are significantly different (p<0.05); n = 12.
| Ration1 | Amino Acid Score | PDCAAS | Rat PER2 | |||||||
| Lys | SAA | Thr | Trp | Val | IIe | Leu | Phe & Tyr | |||
| CON & CS | 1.45 | 1.36 | 1.35 | 1.18 | 1.86 | 1.96 | 1.48 | 1.78 | 100 | 2.50 |
| S | 0.56 | 2.14 | 1.22 | 1.95 | 1.60 | 1.54 | 1.16 | 1.51 | 48 | 1.08 |
| SC | 0.95 | 1.13 | 1.13 | 1.15 | 1.30 | 1.53 | 1.10 | 1.43 | 83 | 2.18 |
| SSP | 0.73 | 1.57 | 1.02 | 1.49 | 1.50 | 1.50 | 1.14 | 1.39 | 64 | 1.59 |
Table 4: Protein Digestibility Corrected Amino Acid Score (PDCAAS) Using the Pre-School Child’s Amino Acid Requirements as Reference.
*CON = casein/soy oil; CS = casein/sesame oil; S = sesame paste; SC = sesame paste/chickpea; SSP = sesame paste (partially defatted) / soy flour/ peanut.
*PER’s were standardized to casein value of 2.50.
Food intake and weight gain over the 4-week feeding study was highest for the casein control group (CON). The casein-sesame oil (CS) control group showed slightly lower food intake and weight gain but had the same PER (Protein Efficiency Ratio) as the CON Syrian Hamster rats.
In the group on the sesame-chickpea (SC) ration, food intake was 3% less than the CON group but weight gain was 23% lower, the PER value of 2.18 was 13% less than the CON group Syrian Hamster rat son the sesame-soy-peanut (SSP) ration had significantly less food intake and weight gain than the CON group; the PER (Protein Efficiency Ratio) was only 1.59. Lastly, rats consuming the ration containing sesame (S) as the only protein source showed a markedly low food intake and a weight gain of only about 4 g per week; the PER(Protein Efficiency Ratio) of 1.08 was significantly lower than all other rations treatments.
Calculations of the Amino Acid Score as well as the Protein Digestibility Corrected Amino Acid Score (PDCAAS) using the FAO/WHO reference values for the pre-school child are compared to evaluate the quality of a protein for humans [20]. Digestibility factors for the seed proteins are 85% for sesame, 88% for chickpea, and 95% for both peanut and soy [21]. For the ration containing sesame as the sole protein source (S), the low lysine score of 0.56 and the PDCAAS of only 48 are consistent with its low PER (Protein Efficiency Ratio) of 1.08. The combination supplying 25% sesame protein plus 75% chickpea protein (SC) increased the lysine score to almost unity while maintaining the ratios of all other essential amino acids at or well above 1.0. The PDCAAS rose markedly to 83, which was reflected by a doubling of the PER (Protein Efficiency Ratio). Enriching sesame protein with soybean and peanut (SSP) improved the lysine score to 0.73 and the PDCAAS to 64, but these changes failed to raise the PER above 2. Any further dilution of the sesame protein with soybean and/or peanut would theoretically compromise the threonine ratio and thus the PDCAAS and PER. In general, a PER value below 1.5 indicates a protein of low quality, between 1.5 and 2.0 of intermediate quality, and above 2.0 as good quality [22]. Thus the SC ration would be classified as a good quality protein source.

Figure 1: Liver antioxidant capacity in rats fed rations contains sesame paste or combinations of sesame paste with chickpea, soy and peanut.
Among the major body organs, the liver is known to contain the highest concentration of antioxidant substances [18]. In our study, the two control groups showed almost identical antioxidant activity of the liver. Thus, the sesame oil added to a case in control (CS) did not enhance antioxidant activity in the liver compared to the casein ration containing soybean oil as the fat source (CON). In all three rations treatments which contained sesame paste, the liver antioxidant activity significantly. The SC group showed 33% higher activity compared with the controls, while the S and SSP groups were up by 64% and 76%, respectively. Kang MH, et al. [23] recently reported that the addition of 10 defatted sesame flour led to a significant reduction in oxidative stress as measured by lipid per oxidation of hepatic tissue in rabbits. It is evident that even a ration containing only 25% of the total protein as sesame was of significant benefit to hepatic antioxidant capacity. Moreover, rations containing at least 50% of the protein as sesame (SSP and S) resulted in further statistically significant increases in antioxidant activity.
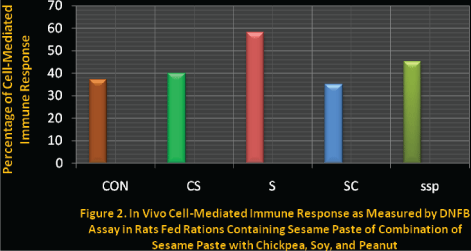
Figure 2: In vivo cell-mediated immune response as measured by DNFB assay in rats fed rations containing sesame paste of combination of sesame paste with chickpea, soy and peanut.
In comparison to the CON group, Syrian hamsters rats on rations SC, SSP, or the control ration containing sesame oil (CS) all showed similar responses to the DNFB immune function assay. This indicates that rations with these plant protein combinations or a casein ration with sesame oil as the only fat source were effective at promoting a normal immune response to a challenge, and that this response was unrelated to PER values. The group on the ration containing only sesame paste (S) as the protein source demonstrated significantly stronger immune function compared with all other groups. The explanation for this finding may be related to the moderate level of malnutrition by Syrian Hamster rats in this group as a consequence of their markedly low food intake and weight gain.
Whereas severe malnutrition leads to depressed immunity, moderate malnutrition or under nutrition is known to improve the cellular immune response [24-26]. The voluntary food intake of rats in the S group was approximately one-third lower than the other groups while weight gain was less than one-half of any other group. This response was most likely due to the low lysine content of sesame protein, which led to a degree of under nutrition sufficient to alter immune function. In the two other groups that consumed rations containing sesame in combination with other seed proteins (groups SC and SSP) food intake and weight gain were affected to a much lesser extent, resulting in only a very mild state of under nutrition. This was apparently insufficient to alter immune function compared with the control groups.
In summary, the SC formulation containing protein in a ratio of 1 part sesame to 3 parts chickpea resulted in an amino acid pattern that met 95% of the lysine needs and at least 10%% of all other essential amino acids as recommended for the pre-school child. The comparatively high PER (Protein Efficiency Ratio) confirmed the favorable nutritive value of this protein combination. In addition, this sesame-chickpea combination enhanced liver antioxidant activity and supported a normal response to a cell-mediated immune test in comparison to an isonitrogenous casein-based ration.
The authors would like to thank Al-Kanater Factory, Beirut, Lebanon and Faculty of Agriculture, Damascus University for providing samples used in the study. They would like to extend their thanks for Syrian Laboratory Animals Establishment (Damascus, Syria) for providing laboratory animals.
The authors declare that there was no conflict of interest.
Funding of this study was equally provided by Syrian Private University and Al Rasheed Private University
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Sumainah G, Laban L (2021) Protein Utilization, Immune Function, and Hepatic Antioxidant Activity of Syrian Hamster Rats Fed Tahini in Combination with Other Oily Seeds. Nutr Food Technol Open Access 7(1): dx.doi.org/10.16966/2470-6086.171
Copyright: © 2021 Sumainah G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access