Abstract
The intake of added sugars, such as high-fructose corn syrup and sucrose, has markedly increased in the last hundred years and is closely
associated with the increased prevalence of obesity, metabolic syndrome, and type 2 diabetes. We recently reported that the glucose- and
fructose-induced generation of glyceraldehyde (GA) caused GA-derived advanced glycation end-products (GA-AGEs), which may be used
biomarkers to predict lifestyle-related diseases. Therefore, we assessed total sugar and glucose concentrations in 885 and 298 commonly
consumed beverages, respectively, in Japan. Our results revealed that total sugar concentrations were markedly higher in some carbonated
drinks, sugar-sweetened fruit drinks, milk beverages, fruit mix juices, milk, cocoa, black tea, fruit juices, and other beverages. Total glucose
concentrations were also higher in some carbonated drinks, sugar-sweetened fruit drinks, fruit mix juices, and fruit juices. About 40% of the
beverages contained 25 g or more sugar per bottle based on standard serving sizes. This is the upper limit of daily sugar intake, recommended
in the guidelines by the American Heart Association and the World Health Organization to prevent health problems in women and adults/
children, respectively. This study has provided potentially useful data on the presence of sugars in commonly consumed beverages.
Keywords
High-Fructose Corn Syrup (HFCS); Sugar-Sweetened Beverages (SSB); Advanced Glycation End-products (AGEs);
Glyceraldehyde (GA); Toxic AGEs (TAGE); Metabolic Syndrome (MetS); Lifestyle-related Diseases
Abbreviations
AGEs: Advanced Glycation End-products; AHA: American Heart Association; CML: Nε-(Carboxymethyl)lysine; CRF:
Chronic Renal Failure; CVD: Cardiovascular Diseases; GA: Glyceraldehyde; GA-AGEs: GA-derived AGEs; GAPDH: G-3-P dehydrogenase;
GFCS: Glucose-Fructose Corn Syrup; Glu-AGEs: Glucose-derived AGEs; G-3-P: GA-3-phosphate; HbA1c: Hemoglobin A1c; HFCS: HighFructose
Corn Syrup; HMG-CoA: 3-Hydroxy-3methylglutaryl-CoA; HOMA-IR: Homeostatic Model Assessment of Insulin Resistance; IR: Insulin
Resistance; JAS: Japanese Agricultural Standard; MetS: Metabolic Syndrome; NAFLD: Nonalcoholic Fatty Liver Disease; NASH: Nonalcoholic
Steatohepatitis; RAGE: Receptor for AGEs; SSB: Sugar-Sweetened Beverages; TAGE: Toxic AGEs; T2D: Type 2 Diabetes; WHO: World Health
Organization
Introduction
The combination of two simple sugars, High-Fructose Corn Syrup
(HFCS) and sucrose, which are used in many Sugar-Sweetened Beverages
(SSB) and commercial products, is commonly consumed worldwide. A
growing body of epidemiological and mechanistic evidence argues that
excessive sugar consumption affects human health beyond the simple
addition of calories [1]. Sugar has been implicated in the development
of all diseases associated with Metabolic Syndrome (MetS) [2,3],
including hypertension, Cardio Vascular Diseases (CVD), Nonalcoholic
Fatty Liver Disease (NAFLD)/Nonalcoholic Steatohepatitis (NASH),
Type 2 Diabetes (T2D) and the ageing process, which is promoted by
damage to proteins due to the non-enzymatic binding of sugars (socalled
glycation) [4-8]. Two major sources of Advanced Glycation Endproducts
(AGEs), exogenous and endogenous AGEs, have been identified
in humans [4-7]. AGEs are formed by the Maillard reaction, a nonenzymatic
reaction between the terminal α-amino group or ε-amino
group of the lysine residues of proteins and the aldehyde or ketone
groups of reducing sugars, such as glucose, fructose, and glyceraldehyde
(GA) [4-7]. We recently demonstrated that interactions between GAderived
AGEs (Toxic AGEs, TAGE) and the Receptor for AGEs (RAGE)
altered intracellular signaling, gene expression, and the release of proinflammatory
molecules and also elicited oxidative stress in numerous
types of cells, all of which may contribute to the pathological changes
observed in lifestyle-related diseases, such as T2D, diabetic vascular
complications, hypertension, Alzheimer’s disease, CVD, NAFLD/NASH,
and cancer growth and metastasis [8-13].
Two different pathways are responsible for the in vivo generation
of GA, which is the precursor of TAGE: i) the glycolytic pathway
(glycolysis) and ii) the fructose metabolic pathway (fructolysis) [10-
14]. In pathway i) the enzyme GA-3-phosphate (G-3-P) dehydrogenase
(GAPDH) generally breaks down the glycolytic intermediate G-3-P.
However, reductions in GAPDH activity lead to the intracellular
accumulation of G-3-P. Therefore, G-3-P starts to be metabolized via an
alternative pathway, causing increases in the concentration of GA and, as
a result, promoting the synthesis of TAGE. Therefore, a positive feedback
mechanism is in operation; namely, the inhibition of GAPDH activity by
GA promotes the synthesis of TAGE. In pathway ii), an increase in
intracellular glucose concentrations under hyperglycemic conditions
stimulates the production of fructose via the polyol pathway in insulinindependent
tissues, such as nerve tissues, the kidneys, the lens of the
eyes, red blood cells, and the brain [15,16]. Fructose is a constituent of
HFCS and sucrose, and, hence, is commonly included in the human diet
[17,18]. Fructokinase phosphorylates fructose to fructose-1-phosphate,
which is then broken down into GA and dihydroxyacetone phosphate by
aldolase B [19,20]. The resultant GA is transported (or leaks passively)
across the cell membrane. GA induces the synthesis of TAGE in the
intracellular and extracellular compartments.
We recently indicated that serum levels of TAGE, but not those of
hemoglobin A1c (HbA1c), glucose-derived AGEs (Glu-AGEs), or Nε-
(carboxymethyl)lysine (CML), a representative AGE compound found in
food, may be used as a biomarker to predict the progression of lifestylerelated
diseases [21-25]. We also reported an increase in the expression
of hepatic RAGE and the enhanced production/ accumulation of TAGE
in normal rats administered Glu-AGE-rich beverages [26]. These findings
indicated that Glu-AGEs, which are normally contained in beverages and
foods [27], and are taken orally into the body, enhance the production/
accumulation of TAGE, leading to TAGE-RAGE interactions. Therefore,
the aim of the present study was to assess the concentrations of total sugars
and glucose, which was metabolized to GA as a precursor of TAGE in
the liver, in commonly consumed beverages in Japan.
Methods
Measurement of total sugar content
Commonly consumed beverages in Japan were purchased from
vending machines, convenience stores, or supermarkets in Kanazawa city.
We classified beverages according to the Japanese Agricultural Standard
(JAS). The contents of total sugars were analyzed in 885 different
commercially available beverages using a digital refractometer (ATAGO
Palette PR-201 α). The contents of sugar in each beverage were based on
the mean value of at least three measurements per sample and expressed
as sugar content (g). These beverages were undiluted or diluted 2- to
10-fold (after controlling for dilution) with distilled water. The contents
of the total sugars were calculated based on standard serving sizes (65
mL-500 mL/bottle) in beverages.
Measurement of glucose content
Glucose contents were analyzed in 298 different commercially available
beverages by the Glucose C II-Test kit Wako (Wako Pure Chemical
Industries, Osaka, Japan) after they were undiluted or diluted 2- to 10-
fold (after controlling for dilution) with distilled water. The contents
of glucose in each beverage were based on the mean value of at least
three measurements per sample and expressed as glucose content (g). The
contents of total glucose were calculated based on standard serving sizes
(65 mL-500 mL/bottle) in beverages.
Results
Total sugar content in common beverages
The average total sugar content in each type of beverage is shown
in ( Figure 1). The numbers of beverages in each category that contained
≥ 50; 25-49.9; 12.5-24.9 and <12.5 g/bottle of total sugars are shown in
(Table 1). The amount of total sugars was ≥ 25 g/bottle in ca. 40% of the
beverages examined. These results suggested the excessive consumption
of beverages, especially carbonated drinks, sugar-sweetened fruit drinks,
milk beverages, fruit mix juices, milk, cocoa, black tea, fruit juices, and
other beverages, needs to be avoided because they contain large amounts
of sugars. On the other hand, oolong tea and tea had low sugar content.
Moreover, products that used an artificial sweetener (such as aspartame,
acesulfame potassium, and sucralose), also in carbonated drinks, also
had a low sugar content.
Glucose content in common beverages
The average total glucose content in each type of beverage is shown
in Figure 2. The numbers of beverages in each category that contained
≥ 20; 10-19.9; 5-9.9; and <5 g/bottle of total glucose are shown in Table
2. The amount of glucose was ≥ 10 g/bottle in ca. 32% of the beverages
examined. These results suggested that the excessive consumption
of beverages, especially carbonated drinks, sugar-sweetened fruit drinks,
fruit mix juices, and fruit juices, needs to be avoided because they
contain large amounts of glucose. On the other hand, black coffee,
coffee, cocoa, health drinks, and soy milk had low glucose content.
Moreover, products that used an artificial sweetener, also in carbonated
drink, had low glucose content. Tea and oolong tea did not contain any
glucose (data not shown).
Calculation of fructose and sucrose contents in common
beverages
Tables 3 and 4 shows the amount of total sugars and free glucose as
well as the calculated fructose/sucrose in a typical beverage in Japan,
among drinks with total sugars over 50 g/bottle. The highest total sugar
and free glucose contents were observed in carbonated drinks with the
commercial names shown in Table 3, followed by sugar-sweetened fruit
drinks with the commercial names shown in Table 4. The other
highest total sugar contents were detected in fruit juices (Max-Min: 61-53
g, 4 kinds), other beverages (60-51 g, 9 kinds), coffee (GEORGIA MAX
COFFEE-X, COCA-COLA, 60 g), fruit mix juices (Oishii 100% mix
juice, SANGARIA, 57 g; Orange-blend 100%, SANGARIA, 55 g), and
lactic acid bacteria beverages (THE PREMIUM CALPIS, CALPIS, 56 g),
which were identified as beverages containing more than 50 g/bottle of
total sugar. The other highest total glucose contents were detected in other
beverages (Calpis water and CALPIS, 21 g/57 g sugar), fruit juices
(Minute Maid Asano-kenkou-kazoku Cassis & Grape 100%, COCACOLA,
20 g/42 g sugar), and fruit mix juices (Vitamin-fruit, Maturegrape,
sugar content 12°C, ITOEN, 20 g/45 g sugar), which were
identified as beverages containing more than 20 g/bottle of total glucose.
Discussion
The increasing number of patients with T2D in Asian countries
including Japan is an important public health problem [28]. One welldocumented
change that may contribute to the risk of T2D in Asia and
elsewhere is the consumption of SSB [29,30]. The increased consumption
of SSB has been observed not only in Western, but also in Asian countries
[31], and has been extensively associated with an increased risk of T2D
and also with weight gain, obesity, MetS, hypertriglyceridemia, coronary
heart disease, and hypertension [32-38]. In the setting of a pandemic of
obesity and T2D, the American Heart Association (AHA) has recently
released scientific recommendations to reduce added-sugar intake to no
more than 100 (for women)-150 (for men) kcal (25-37.5 g sugar)/day
for most Americans [39]. A new World Health Organization (WHO)
[40] guideline has recommended that adults and children reduce
their daily intake of added sugars to less than 10% of their total energy
intake (50 g sugar for a 2,000 kcal/day diet). An additional reduction
to below 5% of the total energy intake or roughly 25 g sugar/day may
provide additional health benefits. This limited is markedly exceeded
by today’s society [41]. About 40% of the beverages contained 25 g
or more sugar per bottle based on standard serving sizes. This is the
upper limit of daily sugar intake, recommended in the guidelines by
the AHA (2009) and the WHO (2015) to prevent health problems in
women and adults/children, respectively (Table 1). A 500-mL bottle of a
carbonated drink (Coke, Sprite, or Fanta) contains approximately 50-
60 g of added sugars; therefore, the consumption of one bottle equals
the recommended amount of added sugars for 1 day. Imamura et al. [42]
prospectively examined the relationship between consumption of SSB
and risk of T2D from 17 cohorts (38,253 cases/10,126,754 person years).
They repeated meta-analysis to estimate the relative risk for each 250 mL/
day. Higher consumption of SSB was associated with a greater incidence
of T2D, by 18% per one serving/day (95% confidence interval 9% to 28%)
and 13% (6% to 21%) before and after adjustment for adiposity. Habitual
consumption of SSB was associated with a greater incidence of
T2D, independently of adiposity. These findings suggested that the
continued intake of negligible amounts of SSB increased the risk of T2D.
The consumption of SSB has been directly and indirectly linked to an
increased risk of T2D. Extensive and lasting changes in public policies are
needed to curb the worldwide obesity and T2D epidemics, and limiting
the consumption of SSB may be an important strategy to achieve this.
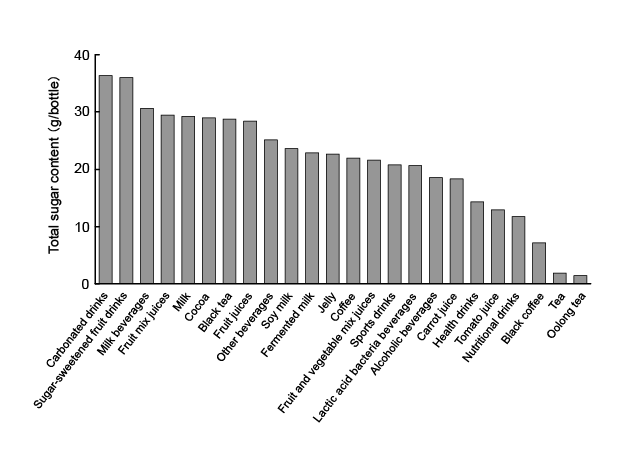
Figure 1: Average total sugar contents in various common beverages. Beverages were classified according to the Japanese Agricultural Standard
(JAS). The average total sugar content in each beverage was based on the mean value and expressed as total sugar content (g) per bottle of beverage
for a standardized serving size.
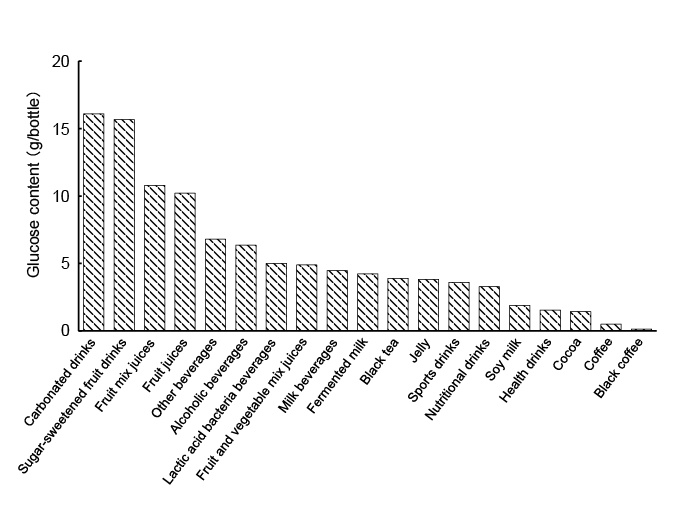
Figure 2: Average glucose contents in various common beverages. The total glucose content in each beverage was based on the mean value and
expressed as glucose content (g) per bottle of beverage for a standardized serving size.
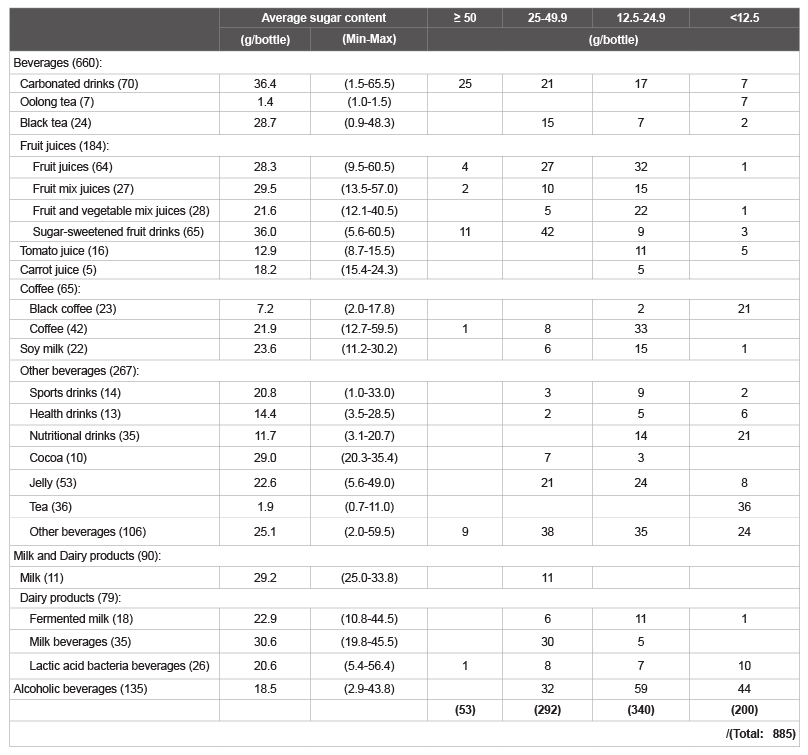
Table 1: The number of common beverages tested for sugar content (Total 885).
We herein showed the amount of total sugar and free glucose and
calculated fructose plus sucrose in a typical beverage in Japan (Tables
1-4). The amount of glucose was ≥ 10 g/bottle (the amount of glucose
that is prescribed during hypoglycemia to diabetic patients in Japan) in
ca. 32% of the beverages examined (Table 2). The highest total sugar
and free glucose contents were observed in carbonated drinks, followed
by sugar-sweetened fruit drinks with the commercial names shown in
Tables 3 and 4, among drinks with total sugars over 50 g/bottle. In
these tables, most SSB (especially those containing HFCS) contained more
fructose than glucose, while glucose-fructose corn syrup (GFCS) contains
more glucose than fructose. Fructose has several metabolic properties that
make it more toxic than glucose. It is a potent stimulant of de novo
lipogenesis, leading to ectopic fat deposition and Insulin Resistance
(IR) [43]. It does not stimulate insulin or the subsequent secretion
of leptin, thereby failing to induce satiety signals [44,45]. Recent
studies showed that the excess consumption of SSB was associated
with obesity, a cardio metabolic risk, CVD, and NAFLD [46-49]. SSB
have been targeted as one of the primary culprits in the escalating
rates of obesity and T2D, and reductions in added sugars have been
considered necessary in order to promote cardiovascular health and
reduce deaths from cardiovascular causes.
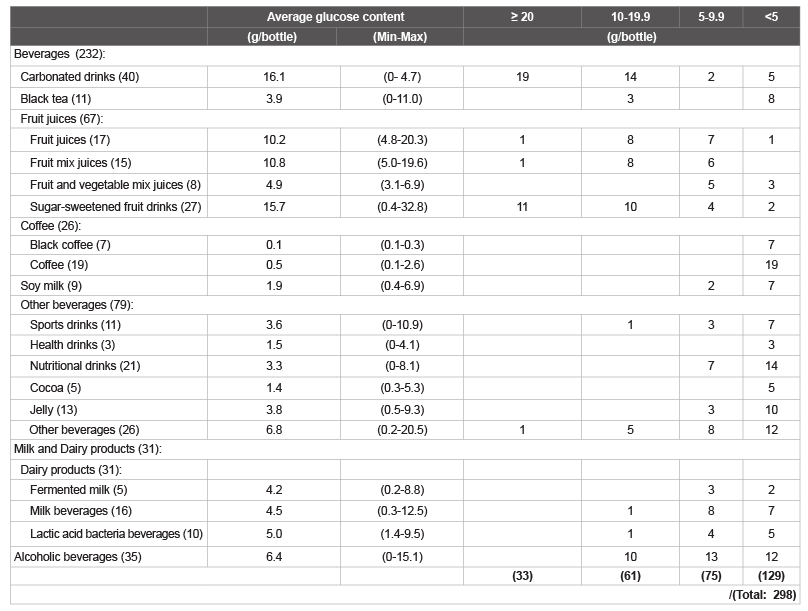
Table 2: The number of common beverages tested for glucose content (Total 298).
The liver is very sensitive to changes in nutrient delivery and is
uniquely suited to metabolize ingested simple sugars, such as fructose
and glucose. Fructose and glucose are metabolized to GA, a precursor
of TAGE in the liver (Figure 3). The findings of our recent studies
revealed that i) the formation of TAGE was enhanced during NASH,
and serum and hepatic TAGE levels, but not Glu-AGEs and CML, were
significantly higher in patients with NASH than in healthy controls
or patients with simple steatosis [50], ii) atorvastatin, a 3-hydroxy-
3-methylglutaryl (HMG)-CoA reductase inhibitor, reduced serum TAGE
levels in NASH patients with dyslipidemia [51], iii) the serum level of
TAGE, but not CML, was one of the independent correlates in the
homeostatic model assessment of IR (HOMA-IR) in non-diabetic
subjects [21], iv) TAGE, but not HbA1c or Glu-AGEs, may be
used as a biomarker to reflect cumulative postprandial hyperglycemia
in T2D patients [22], v) the level of TAGE, but not HbA1c or CML, was
independently associated with vascular inflammation, as evaluated by
[18F] fluorodeoxyglucose-positron emission tomography (FDG-PET) in
outpatients [23], vi) TAGE levels were one of the independent correlates of
the decreased cell number and impaired migratory activity of circulating
endothelial progenitor cells in apparently healthy subjects [24], and vii)
high baseline TAGE levels were associated with plaque progression in
an assessment of pitavastatin and atorvastatin in an acute coronary
syndrome trial (The JAPAN-ACS Sub-study) in Japan [25]. These findings
indicated that the serum level of TAGE, but not HbA1c, CML, or GluAGEs,
may be used as a biomarker to predict the development and
progression of lifestyle-related diseases. SSB need to be taken into
consideration for disease prevention, particularly in individuals at high
risk of developing lifestyle-related diseases.
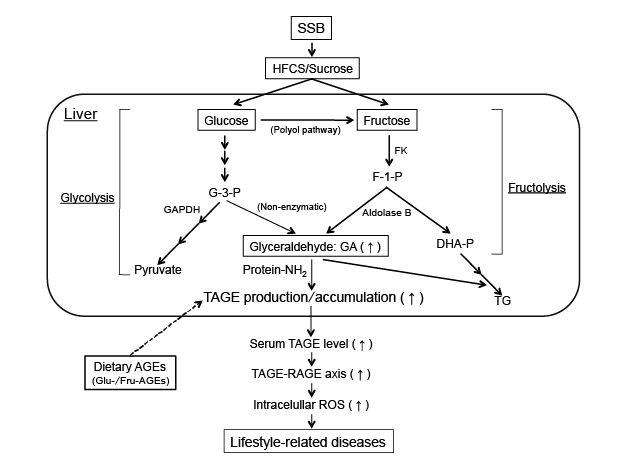
Figure 3: Effects of the commonly consumed dietary sugar metabolite, glyceraldehyde (GA). The chronic ingestion of excessive amounts of SSB
(HFCS/sucrose) increases the levels of the sugar metabolite, GA in the liver. GA is known to react non-enzymatically with the amino groups of proteins
to form GA-AGEs (TAGE). The interaction between TAGE and RAGE alters intracellular signaling, gene expression and the release of pro-inflammatory
molecules and also elicits the generation of ROS in numerous types of cells, all of which may contribute to the pathological changes observed in
lifestyle-related diseases [8-13]. Furthermore, the chronic ingestion of excessive dietary AGEs (mainly Glu-/Fru-AGEs) increases the expression of
RAGE and the enhanced production/accumulation of TAGE, thereby leading to TAGE-RAGE interactions.
AGEs: Advanced Glycation End-products; DHA-P: Dihydroxyacetone-phosphate; F-1-P: Fructose-1-phosphate; FK: Fructokinase; G-3-P:
Glyceraldehyde-3-phosphate; GA: Glyceraldehyde; GA-AGEs: Glyceraldehyde-derived AGEs; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase;
HFCS: High-Fructose Corn Syrup; RAGE: Receptor for AGEs; ROS: Reactive Oxygen Species; SSB: Sugar-Sweetened Beverages; TAGE: Toxic
AGEs; TG: Triglyceride; Protein-NH2: Free Amino Acids of Proteins.
We previously reported that the hepatic expression of RAGE was
elevated in normal rats that had been given a Glu-AGE-rich beverage
[26]. In addition, hepatic cells were found to contain TAGE and GluAGEs
even though the beverage administered to these rats did not
contain TAGE. These findings suggest that the synthesis and hepatic
accumulation of TAGE are promoted by Glu-AGEs, which are often
found in SSB and foods [27], resulting in increased TAGE-RAGE
interactions [26,52]. In another study, Kremezin, an oral adsorbent that
slows the development of chronic renal failure (CRF) by promoting
the removal of uremic toxins, reduced serum Glu-AGE and TAGE
concentrations in non-diabetic CRF patients [52]. The chronic ingestion
of excessive amounts of SSB, which contained HFCS, sucrose, and
dietary AGEs, increased the levels of the sugar metabolite, GA in the
liver. GA is known to react non-enzymatically with the amino groups
of proteins to form TAGE, enhance the production/accumulation of
TAGE, up-regulate RAGE mRNA levels, and increase serum TAGE levels,
leading to TAGE-RAGE interactions. The interaction between TAGE and
RAGE has been shown to alter intracellular signaling, gene expression
and the release of pro-inflammatory molecules and also elicit the
generation of reactive oxygen species in numerous types of cells, all of
which may contribute to the pathological changes observed in lifestylerelated
diseases (Figure 3) [8-13]. The results of the present and previous
studies suggest that sugars (glucose, fructose, and sucrose) are present in
appreciable levels in common beverages, and exogenous, dietary GluAGEs
[27] may contribute to the accumulation of TAGE in the body.
The contents of HFCS/sucrose and dietary AGEs in beverages/foods
need to be taken into consideration for disease prevention, particularly
in individuals at high risk of developing lifestyle-related diseases.
Conclusion
We here presented useful information regarding the sugar
concentrations of numerous beverages that are commonly consumed
in Japan. Much of the sugars consumed today are “hidden” in processed
foods that are not regarded as sweets. Added sugars refer to HFCS and
sucrose added to drinks and foods by the manufacturer, cook, or
consumer, and sugars naturally present in honey, syrups, and fruit juices.
Current social and environmental factors have been linked to the purchase
and consumption of SSB, including advertising and promotions, increased
portion sizes, fast food consumption (at convenience stores, supermarkets
(food markets), restaurants, and vending machines), television watching,
permissive parenting practices, parental SSB consumption, sneaking
away-from-home meals, and increased access to SSB in the home and
school [53]. Thus, free fructose consumption may be mainly responsible
for the cardiovascular risk associated with SSB worldwide, and their
adverse metabolic effects may also be related to their fructose over glucose
fraction [54]. Additional clinical investigations may provide us with
more information as to whether the restriction of dietary sugars and GluAGEs
is beneficial for the prevention and progression of lifestyle-related
diseases and may be a novel therapeutic target to prevent these diseases.
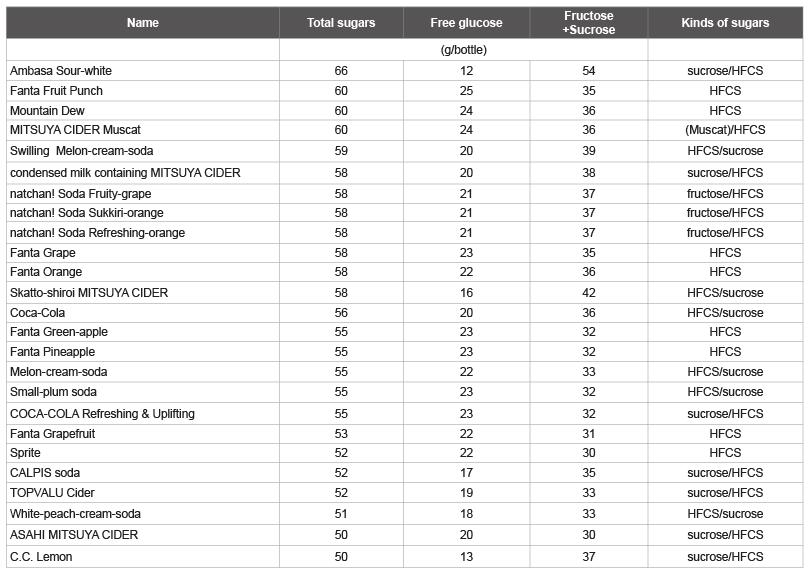
Table 3: List of carbonated drinks containing total sugar, free glucose, and fructose plus sucrose
HFCS: High-Fructose Corn Syrup
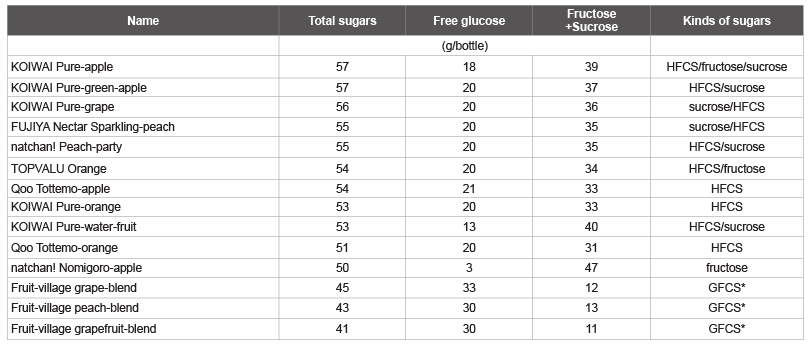
Table 4: List of sugar-sweetened fruit drinks containing total sugar, free glucose, and fructose plus sucrose
*GFCS: Glucose-Fructose Corn Syrup
Acknowledgments
This study was supported by grants from the Japan Society for
the Promotion of Science (JSPS) (KAKENHI Grant Numbers 22300264
& 25282029 for M.T.).
Conflict of interests
The authors declare no potential conflicts of interest.








