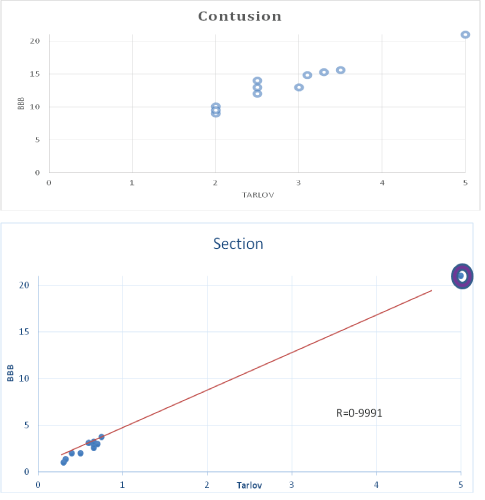
Figure 1: Correlation between BBB and Tarlov scales in control group with contusion (a) section (b) both with no treatment. The positive correlation coefficients are very high ˃.95 with significance p˂0.01 Sham is represented with


Carlos Torres-Vega* Ana Edith Higareda-Mendoza Marco Aurelio Pardo-Galván
Celular Biology Lab, Faculty of Medicine, UMSNH ( Universidad Michoacana de San Nicolas de Hidalgo), Mexico*Corresponding author: Carlos Torres-Vega , Celular Biology Lab, Faculty of Medicine, UMSNH ( Universidad Michoacana de San Nicolas de Hidalgo), Mexico, E-mail: carlos.torresvega@gmail.com
Multiple therapeutics looking for functional motor reconnection in spinal cord injury has been reported with unpromising results; they are not yet acceptable for clinical application. They have been studied individually or in combinations looking for synergy mostly on therapeutic attempts of chronic injury and on few experimental acute injury models.
Materials and methods: A model of acute spinal cord injury on mice was used, with application of heterologous Olfactory Bulb Graft (TBO) and intraperitoneal systemic drugs: paclitaxel (T), Ambroxol (A) and Colchicine (C), Applied Individually (TA) (TAC) or in combination (TBO-TA) (TBO-TAC) compared with control groups without treatment. Motor recovery was assessed with a simplified Tarlov’s scale and BBB (Basso, Beathie, Bresnahan) scale, at baseline and up to 12 weeks post-injury, done for to blind evaluators. Video-Tracking process with the Tox Track program was made, measuring parameters of speed, acceleration, and lifting’s on hind limbs. A histological study was performed with light and transmission electron microscopy. Results were analyzed by comparing standard means and errors, as well as percentage proportions using the SPSS 25.0 package.
Results: Very significant motor recovery was demonstrated at 12 weeks in the groups combining TBO and triple drug (TBO-TAC). In the groups with TBO and only drugs separately, recovery was shown on less degree; but greater than controls. The Sham group had no motor deficit. Histological evidence of increased myelination was obtained in combination therapy.
Conclusions: The results indicate that all the treated groups, TBO, drugs and the combination of both were superior to the controls, being more important and spectacular in the group with combination.
Spinal cord; Injury; Olfactory bulb; Transplant; Paclitaxel; Ambroxol; Colchicine; Motor recovery
Since the publication of y Cajal SR, et al. on the degeneration and regeneration of the nervous system [1], accepting as a dogma that the no axonal regeneration on central brain or medullary neurons, it was hinted that under special circumstances this process could occur in central neurons. It took almost 70 years to restart research in this regard [2]. In the last 4 decades there have been advances and research that could promisingly change the dogmatic position that spinal cord injury is irreversible and achieve the process of axonal regeneration and functional recovery in central neurons, which would have a great clinical impact when talking about spinal cord injuries [3-8].
The use of cell therapy for the treatment of spinal cord injury began experimentally 20 years ago, with promising but uncertain results [9-11]. In a review of all the works using olfactory ensheathing glia cells [12] with diverse varieties of taking and application, ranging from complete olfactory bulb transplantation, to purification of subvarieties of glia cells of the nasal mucosa, or peripheral region of the bulb, results are variable and only partial. This olfactory ensheathing glia transplant approach would work in several ways [13,14], with local production of neurotrophic factors regenerating axonal and differentiation, inhibition and neutralization of inhibitory factors in the microenvironment such as chondroitin sulfate proteoglycans and proteins associated with myelin [9], as well as with the proliferation of adult neurogenic stem cells of the ependymal epithelium [15,16].
This has led to searching for alternative pharmacological therapies to stimulate possible axonal regeneration, since its intrinsic mechanisms have been partly elucidated: a numb program of axonal regeneration, the breakdown of microtubules, inhibitory factors in the perineuronal network etc., which prevent the axonal regeneration of central neurons [17,18] and some alternatives to reverse these mechanisms: reprogramming neuronal activity for regeneration, stabilization of microtubules, inhibition of inhibitory factors in the perineuronal medium and stimulation of actin dynamics. Drugs such as: Paclitaxel (Taxol), Epothilone D and B, which stabilize microtubules by their interaction on Beta fraction of tubulin and affinity to microtubule stabilizing Tau proteins [19-21]. These drugs have been evaluated individually on single axonal lesion models of metazoans such as Caenorhabditis elegans, on in vitro models of neuronal cultures and in rodent models of optic nerve injury, as well as spinal cord section, with encouraging results [22-29]; but there are no works with the combination of them. In addition, these works are mainly focused on evidencing morphological proliferation, many of them not having an evaluation of the sensory-motor functional recovery of the animal. Ambroxol, which stimulates a large number of genes and signaling pathways of axonal regeneration was proposed and evaluated by Chandran V, et al. [30] on a mouse model of optic nerve elongation. This has not been studied in spinal cord injury. On the other hand, colchicine, a drug with multiple actions: anti-inflammatory, inhibition of oxide-nitric synthase, as well as destabilizing microtubules (action in apparently contrary to paclitaxel and epothilones), has been studied in models of peripheral nerve injury, with possible actions to stimulate the dynamics of actin, and in the positive segments of microtubules [11,16,18,31,32] important and adjuvant action in the development of growth buttons and neuritic elongation. This has never been studied in medullary section models.
8 groups of 10 animals each were studied, CD-1 strain mice of 5 to 7 weeks of life, with average weight of 35 g (range of 33-42 g) all female non pregnant were used, and managed accordingly with the NOM zoo-1998 of our country that is in accordance to EU Directive 2010/63 of the NIH at USA. Free feed with mouse pellets and climatized two weeks prior to experimental manipulation. Five experimental groups: Transplant of Olfactory Bulb (TBO) alone, Taxol-ambroxol alone (TA), Taxol, Ambroxol and Colchicine (TAC), association of bulb transplantation and drugs in the (TBO-TA) and (TBO-TAC) schemes (Table 1). Two control groups: untreated section and untreated contusion and one Sham group with laminectomy without injury.
| Group | Number of Animals |
| Sham | 10 |
| Section/Not Tx (without treatment) | 10 |
| Contusion/Not Tx (without treatment) | 10 |
| TBO* | 5 section 5 contusion |
| TA** | 5 section 5 contusion |
| TAC*** | 5 section 5 contusion |
| TBO-TA | 5 section 5 contusion |
| TBO-TAC | 5 section 5 contusion |
Table 1: Sham, control and experimental groups.
*TBO-Olfactory bulb transplantation
**TA-Taxol, ambroxol
***TAC-Taxol, ambroxol and colchicines
All animals underwent laminectomy at level T 10, with surgical microscope and microsurgery instruments. The surgeries were performed under intraperitoneal anesthesia with ketamine (2.5 mg/kg)-Xylazine (1.6 mg/kg) on thermal plate at 34° to avoid hypothermia. In the sham group there was no spinal cord injury, it was only washed with saline and calcium gluconate 1%. On control groups the spinal cord was completely sectioned with micro scissors or contusion was performed with Impactor graduated to 1000 dines on 1 single blow, followed by hemostasis and washing with saline and calcium gluconate1% and the aponeurotic muscle plane was closed for stabilization.
On experimental groups, half (5 animals) were sectioned and half (5 animals) expose to contusion with the same parameters indicated. In all groups, prior to the section or contusion a drop of 2% lidocaine was applied for 30 seconds followed by washing with isotonic saline and calcium gluconate, after the section or contusion hemostasis was performed with cotton tip impregnated with hydrogen peroxide a few seconds and a quarter of a heterologous olfactory bulb of a donor mouse obtained 20 minutes before and preserved in sterile saline solution was implanted on TBO-groups. Implant was put directly inside de section line or over the contusion zone. The musculoaponeurotic plane was closed with catgut 4/0 and the skin in another plane with the same material. The animals remained in recovery boxes heated to 27°Celsius for a period of 3 hours. At their recovery (20 minutes in average), paclitaxel 0.5 µg/g, Colchicine 0.01 µg/g and Ambroxol 30 µg/g were administered intraperitoneally as appropriate scheme for each group. For the next 10 days, intraperitoneal ambroxol was administered daily at the same dose of 30 µg/g and at 10 days a second dose of paclitaxel and colchicine of equal magnitude to the first (0.5µg/g and 0.01 µg/g respectively) was repeated as appropriate scheme for each group.
Simplified Tarlov and BBB motor scale assessments were performed in all animals by two blind evaluators using the video recordings at baseline (1 day prior to surgery) 24 hrs post surgery and at 1,2,4,8, and 12 weeks of follow-up. This was done in open ground in arena of 60 × 60 cm. And 1-minute video filming was made, which in addition to serving as evidence to the motor evaluation scale, was analyzed using the Tox-Track program of free access [33] which yields results such as: distance traveled, acceleration and average speed. The number of hind limbs lifts was recorded with the Program True-Scan version 2.0 (Coulbourn instruments, Massachusetts USA).
At 12 weeks after the last motor evaluation and a video recorder of motor activity, animals were sacrificed; the spinal cords were dissected and fixed in Formaldehyde and Glutaraldehyde/Osmium Tetroxide for inclusion in paraffin and resin respectively. HyE and Nissl stains on 10 micron slices, Golgi-Cajal stains were made in thick cuts of 60 microns in longitudinal direction. And 60 nm ultrafine transverse cuts were made from the injury site for electron microscopy.
Both scales were subjected to Spearman correlation test .The motor recovery values were statistically compared with ANOVA and Tukey’s post hoc test and decreases in motor skills at 12 weeks were compared with chi square.
The histological results were obtained on semicuantitative way with the observation of 5 fields to strong dry and 5 segments of N-100 grid of electron microscopy, they were semiquantitative, qualified from 0 to 4 crosses as observed (0 = Not observed) (+= the minimum observed) (++++= the maximum observed). For the degree of myelination seen in TEM, the Fiji image processor was used with a protocol of comparison of ultra microphotographs at 4K magnification, with mask and green-network LUT, quantifying the signal strength in myelin red. 100% myelination was arbitrarily assigned to the Sham group and the percentages of the control and experimental groups were obtained.
Of the 80 animals intervened, we had 1 loss in each of the control groups (2 animals) and in each of the experimental groups (5 animals). In the Sham group there were no losses. There was a very high positive correlation with statistical significance when comparing the scores obtained with the simplified Tarlov’s scale and the BBB (Figure 1).

Figure 1: Correlation between BBB and Tarlov scales in control group with contusion (a) section (b) both with no treatment. The positive correlation coefficients are very high ˃.95 with significance p˂0.01 Sham is represented with
In the follow-up of motor recovery, the sham group showed practically no motor deficit, only in one animal possibly due to injury of a root was there a decrease of 1 point on the Tarlov scale and 3 on the BBB scale, so the average decreased one and two tenths on the respective scale 4.9 and 20.8 and so it remained until week 12. As it can be seen at 24 h post intervention, either the control as the experimental groups had a serious decrease on motor activity, and is at the first week when it is appreciated the initial change to recover. In the contusion groups, both experimental and controls with both evaluation scales had notorious deficit at 24 h and a moderate recovery from 1st week towards the 12th, greater in the experimental groups than in the control. Being higher in the groups that associated bulb transplantation and double or triple drug (4 and 4.3 in Tarlov’s and 16.5 an 17.6 in BBB) only on them was demonstrated statistically significant difference p<0.05 compared to controls (Figure 2).
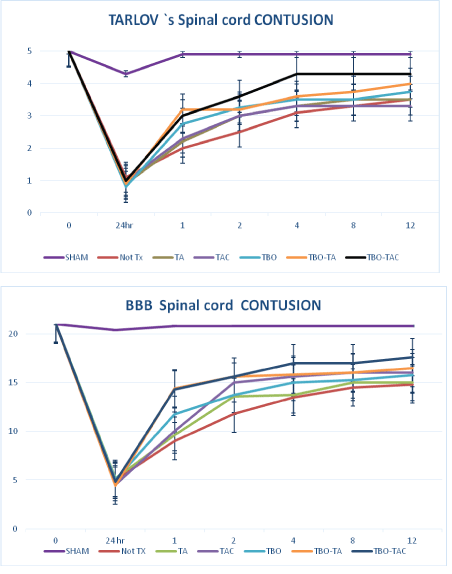
Figure 2: Motor recovery in the contusion group with tarlov scale (a) and BBB scale (b). *only the TBO-TA and TBO-TAC groups showed a significant difference of p˂0.01 compared to controls.
**TBO-TAC had a significant difference with respect to the other treatment group’s p˂0.05
No Tx- without treatment (bars of SE).
In section all groups either controls and treated groups had an almost total motor deficit at lower limbs. The neurological deficit of on control group persisted almost total, averaging 0.75 on the Tarlov’s scale and 1.30 in the BBB at the first week, with persistence practically equal to 12th week. While the experimental groups presented greater recovery in all cases with a significant difference (p<0.01) with respect to controls, being the maximum for the groups with TBO and double or triple drug that reached score in Tarlov’s of 3.5 and 3.8 and BBB of 15 and 16 respectively. Both groups associated with TBO and double and triple drug had a significant difference with respect to the other treatment groups (Figure 3).
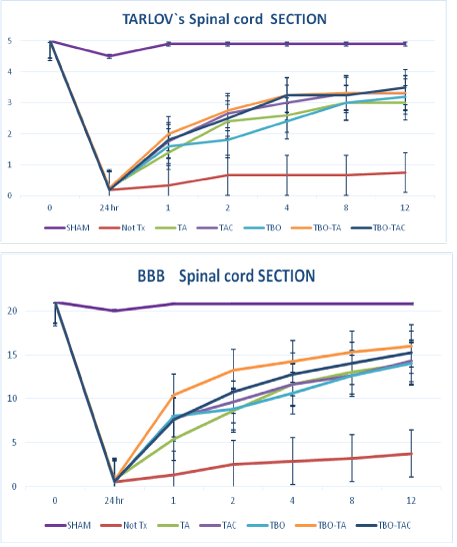
Figure 3: Motor recovery in section groups with Tarlov’s scale (a) and BBB scale (b).* all treatment groups had a significant difference with respect to control-Not Tx.** TBO-TA and TBO-TAC had significant superiority p˂0.05 over other treatment groups.
If we combine section and contusion groups as “Injured group” we can make evident the effect of treatment respect to not treatment groups, either on complete (section) and uncompleted (contusion) spinal cord injury, this represents the clinical variability seen on human medicine, especially the TBO-TAC combination with the best results on both scales. Achieving a 4 in Tarlov’s and 17 in BBB with great statical significance (p<0.01) with respect to the other groups of treatment (Figure 4).
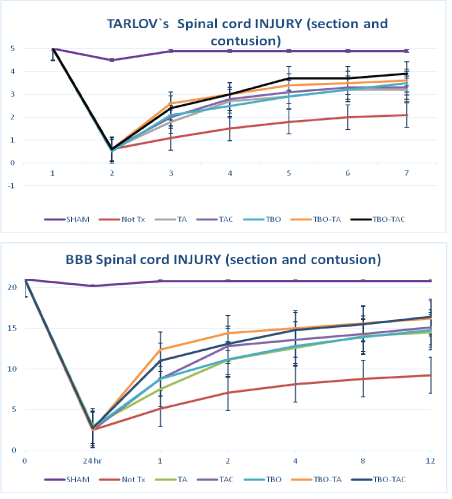
Figure 4: Spinal cord injury (together groups, section and contusion), comparison with Tarlov scale (a) and BBB scale (b). *The differences are statistically significant p˂0.01. With respect to the control group. **The TBO-TA and TBO-TAC groups had a significant difference p˂0.05 compared to other treatment groups. LESION-STX= Injured without treatment. Bars of SE.
A 1-minute video was made in the open field, analyzed with the free access program ToxTrack (Rodriguez A, et al. 2018 [32-33]). We showed only by simplification the results at 12th week of the sham, controls and the TBO-TAC groups that was the one with the best results, the other treatment groups also presented superiority with respect to controls (Figure 5). The results are shown in percentage of decrease at the 12th week with respect to baseline, since this was a common result even in the sham group, however the most intense percentage of decrease occurred in the groups without treatment being 80% in section and 60% in contusion, and was minimized in the treated groups section TBO-TAC 40% and contusion TBO-TAC only 10%. The sham group showed a decrease of only 6.5% (Figure 6).
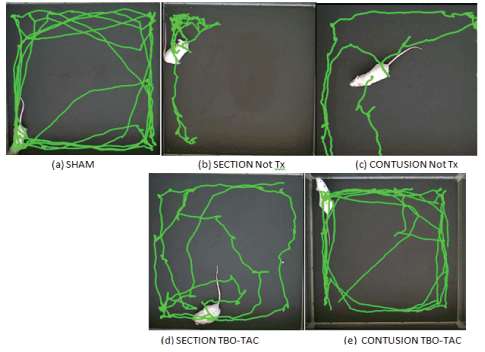
Figure 5: Image of routes of an animal of control groups (No Tx) and TBO-TAC groups at week 12 in Tox Track note the greatest distance of routes and crossings in the treated groups (d and e) with respect to the controls (b and c).
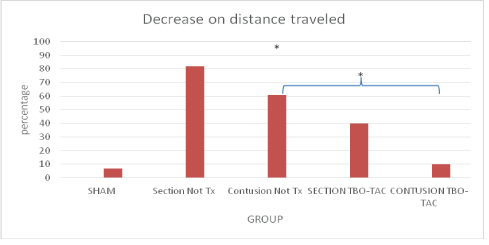
Figure 6: The treatment groups have a lower percentage of decrease on distance traveled that control. *p˂0.05 all compared with basal distance.
As for the number of lifts sustained on hind legs at 12th week, the average on sham group was 6, section without treatment was 0, on contusion without treatment of 1.4, and on the groups with TBO-TAC treatment, 3 for contusion and 2.6 for section (Figure 7).
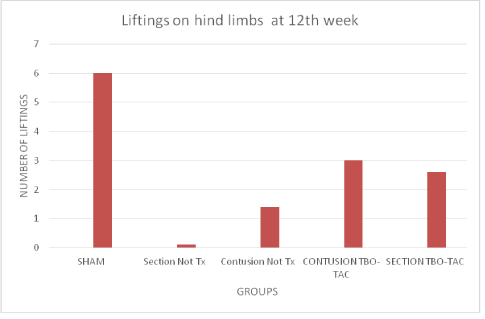
Figure 7: Number of lifts on hind limbs, control groups without treatment do not present lifting’s in section and only 1 in the contusion group, the treated groups reach an average of 3 and 2.6 in contusion and section respectively.
Only the results of sham, control and TBO-TAC groups that had the best results are presented for simplification, mentioning that the other treatment groups also presented superiority to the controls but not greater than the TBO-TAC group . The histological results in light microscopy, using stains, H and E, Nissl and Golgi-Cajal are shown in table 2 and as we can see the treatment group with bulb and triple drug, showed lower degree of gliosis, greater number of neurons preserved and an important number of neurites crossing the site of injury, compared to the control group without treatment that presented significant gliosis, practically without neurites crossing site of injury and almost no neuron preserved.
| Group | Gliosis | Neurons preserved | Neurites crossing line of section |
| Sham | - | ++++ | ++++ |
| Section no tx | ++++ | - | - |
| Contusion no tx | ++ | ++ | ++ |
| Contusion tbo-tac | + | +++ | +++ |
| Section tbo-tac | + | +++ | +++ |
Table 2: Results of microscopy (Hy E, Nissl and Golgi-Cajal) 12th week (Figures 8-10).
They are also illustrated in figures 8-10 with different microphotographs corresponding to each group and comparing them to the sham group with the different stains or impregnation.
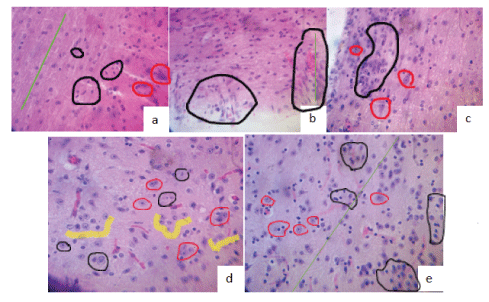
Figure 8: H y E stain at 12th week.
(a) Sham: integral cords (green line), normal glia (black circles), and neuronal bodies (red circles).
(b) Section No Tx: gliosis intense (black circles), absence of neurons, discontinuous cords (green line).
(c) Contusion No Tx: significant gliosis (black circles), few viable neurons (red circles).
(d) Contusion TBO-TAC : minimal reactive gliosis (Black circle), non-reactive glia satellite(yellow) (Implanted cells?) To neurons (red circles), neovascularization, continuity of cords.
(e) Section TBO-TAC: little more reactive gliosis than in d (black circles), with non-reactive glia (yellow) an revascularization. Continuity in laces (green line).
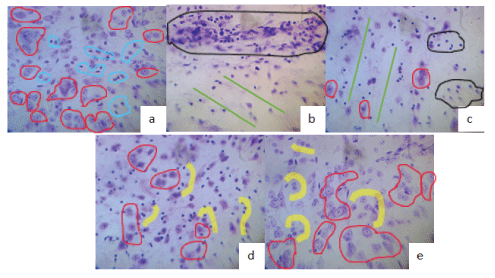
Figure 9: Nissl staining at 12th week.
(a) Sham: Normal neurons preserved (red circles, normal glia).
(b) Section No Tx: complete cord disarray green lines), intense gliosis (black circle), absence of neuronal bodies.
(c) Contusion No Tx: moderate gliosis black circles, few atrophic neurons (red circles), disrupted laces (green lines).
(d) Section TBO-TAC: greater number of non-atrophic neurons (red circles), with respect to groups without Tx, lower gliosis and satellite glia (transplanted olfactory glia?) (Yellow).
(e) Contusion TBO-TAC abundant normal neurons (red circles), little gliosis and satellite glia.
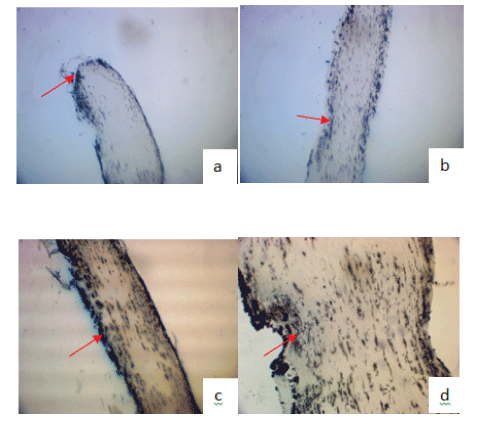
Figure 10: Argentic impregnation, Golgi-Cajal at 12th week.
(a) Section No Tx: proximal end at site of injury (Arrow) amputee.
(b) Contusion No Tx: injury site (arrow) few neurites crossing (black).
(c) Section TBO-TAC greater number of neurites crossing (black) site of injury (arrow).
(d) Contusion TBO-TAC more neurites crossing (black) site of injury (arrow).
The results of the examination in electron microscopy (TEM) of cross-sections of spinal cord injury site are summarized in table 3, where the null or minimal remyelination in the control groups (section and contusion) is frankly seen, and the important remyelination in +++ in the treatment groups.
| Group | Degenerative globes | Myelination |
| Sham | - | (Integral Myelin) ++++ |
| Section not tx | ++++ | - |
| Contusion not tx | +++ | + |
| Contusion tbo-tac | + | +++ |
| Section tbo-tac | ++ | ++ |
Table 3: Results on Transmission electron microscopy (Figures 11 and 12).
Some ultra micrographs in are shown in figure 11 and their analysis with the Fiji Image G program, it is exemplified in figure 12, where the density of the GREEN-RED LUT mask can be observed, red determines the myelin and on the underlying graphic of the same figure the corresponding percentage of myelination is shown, arbitrarily assigning 100% to the sham group. Degree of remyelination was greater in the treated groups than in the controls with Not Tx.
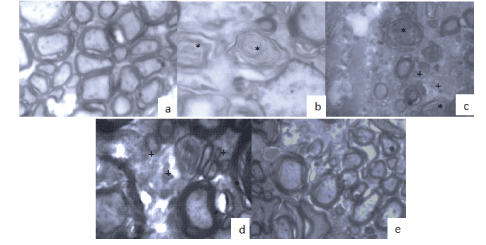
Figure 11: TEM Transverse cut of spinal cord at the level of the site of injury at 12th week 400x.
(a) Sham group, the integrity of the axons and their myelin sheath is maintained.
(b) Not Tx section: axon degeneration and severe demyelination (*).
(c) Not Tx contusion: some degenerative and demyelinated balloons (*), with small remyelinated phyllo pods (+).
(d) Section with Tx TBO-TAC: greater degree of myelination is observed with developed axons and phyllo pods (+).
(e) Contusion with Tx TBO-TAC: higher degree of myelination and number of axons preserved.
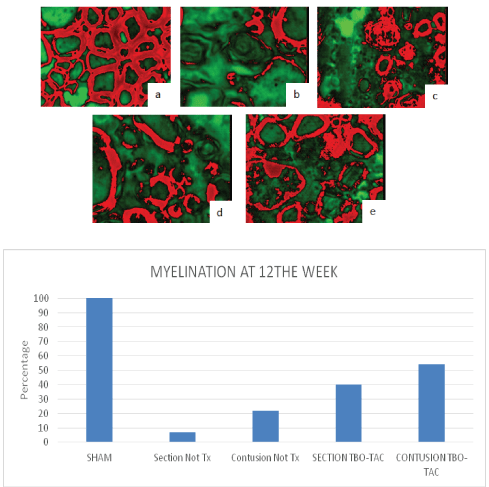
Figure 12: LUT mask red-green myelin in red, analysis with Fiji Image j, sham arbitrarily 100% (a), control groups without treatment little myelination (b and c) 7 and 20% and groups with TBO-TAC treatment greater remyelination (d and e) 40 and 55% (bar graph).
In the present study we clearly demonstrate a motor recovery response and a histological support for it, using the combination of therapeutics previously described individually. As we pointed out in the introduction, the mechanisms specific to the implantation of olfactory ensheating glia cells contained in the heterologous olfactory bulb transplant include the release of neurotrophic factors, the presence of adhesion molecules that would serve as a guide for neurites elongation, the production of metalloprotein lytic enzymes, capable of inhibiting chondroitin proteoglycans, and other inhibitors in the perineuronal network such as Nogo and IMAG proteins, as well as a possible neurodifferentiation of ependymal cells [15]. But also adding a drug such as paclitaxel (taxol) with microtubular stabilization, preserving the fast transport pathways of the axonal body in injured axons [18] and stimulating the axonal regeneration gene program with Ambroxol [30] undoubtedly show potentiation or at least addition as therapeutic synergy.
Colchicine, a drug with diverse, anti-inflammatory effects known through various mechanisms such as inhibition of oxidenitric synthase, microtubular destabilization and others, would appear to be a drug that opposed the effect of paclitaxel, but nevertheless, at small doses, it could be considered adjuvant in the dynamics of microtubules in its positive polar zone, as well as a potential participant in actin dynamics, both processes necessary for the dynamism and elongation of an axonal growth cone, with these concepts and only with indirect evidence [11,16,31,32] We decided to consider its use. The similar or superior results of the association of colchicine to placitaxel and ambroxol, demonstrate its synergistic role and rule out the premise of action contrary to placitaxel.
Our 12-week study shows significant motor recovery, clarifying that we did not subject the animals to any physiotherapy, except for spontaneous mobility in a large cage. This is evident when demonstrating that although motor skills recover, the fact that there is a decrease in distance traveled, with respect to the basal, would show that more time and physiotherapy could improve performance.
Comparing our results with other experimental works, we found clear superiority to what was reported by Arellanes-Chávez CA, et al. [34] and Ramón-Cueto A, et al. [9] and summarized in table 4.
| Author | N | Model | Therapy | Control | BBB | Tarlov |
| Arellanes-Chávez CA, et al. [34] | 6 | Rat/contusion | TBO | Sin control | -------- | 3/5 |
| Torres et al. 2021 | 5 | Mouse/contusion | TBO | Sham/No Tx | 16/21 | 3.8/5 |
| Torres et al. 2021 | 4 | Mouse/contusion | TBO/TAC | Sham/No Tx | 17.6/21 | 4.3/5 |
| Ramón-Cueto A, et al. [19] | 9 | Rat /section | Células GEO | Sham/No Tx | (12/21)* | 2-3/5 * |
| Torres et al. 2021 | 4 | Mouse/section | TBO / TAC | Sham/No Tx | 16.4/21 | 3.8/5 |
Table 4: Comparison of results with other series.
GEO-Ensheating olfactory glia
No Tx-Without treatment
*Calculated
We can say that as it has already been recently disclosed by the great experts in the area [10,18,35,36] the effective therapeutic to achieve the functional recovery of a spinal cord injury, must be the use of combination of synergistic therapies, the present work and the authors, think that this should also be done at the acute moment to achieve the best possible results. Our findings open the possibility to plan a phase I clinical study with this therapeutic.
Finally, we cite a non-recent published review work, in 2007, by Anderberg L, et al. [37], from Uppsala University in Sweden, who titled: “Spinal cord injury: scientific challenges for an unknown future”, where they raise the need and relevance of using combination therapies, and to build bridges between basic science and clinicians and surgeons to solve in a real way the functional recovery of the injured spinal cord. Our work has been an attempt to initiate the responses to these challenges and change the clinical reality in a positive way to a future that has already reached us.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Torres-Vega C, Higareda-Mendoza AE, Pardo-Galván MA (2022) Association of Olfactory Bulb Transplantation with Systemic Drugs: Paclitaxel (Taxol), Ambroxol and Colchicine in a Mouse Acute Spinal Cord Injury Model: Summatory Synergy. J Neurol Neurobiol 8(1): dx.doi. org/10.16966/2379-7150.182
Copyright: ©2022 Torres-Vega C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access