Table 1: Criteria of level of reflex patterns development.

Full Text
Masgutova S1,2* Koberda JL3 Shackleford P1 Nowak K2,4 Akhmatova N1,5 Radchenkov N6 Boldyrev A1 Malova O6,7
1Svetlana Masgutova Educational Institute for Neuro-Sensory-Motor and Reflex Integration (SMEI), LLC, FL, USA2International Dr. Svetlana Masgutova Institute, LLC, Warsaw, Poland
3Tallahassee Neuro-Balance Center, LLC, Tallahassee, FL, USA
4Medical University of Bialystok, Poland
5Mechnikov Institute of Vaccines and Serums, Moscow, Russian Federation
6MNRI Reflex Neuromodulation, SMEI Branch in Moscow, Russian Federation
7Application Neurology Technologies, Co., Moscow, Russian Federation
*Corresponding author: Svetlana Masgutova, Ph.D. in Psychology, Assistant Professor, 6275 Hazeltine National Drive, Orlando, FL 32822, USA, Tel: 608-235-9084; Email : sally@masgutovafoundation.org
This study provides data on MNRI and its neuromodulation effect on: 1) the brain wave spectrum of children with cerebral palsy (CP), 2) regulation of their neurotransmitters, and 3) changes in reflex development level evaluated by the MNRI reflex assessment. Seventy-eight individuals aged 2-19 years (M ± SD=7, 65 ± 4, 85) diagnosed with cerebral palsy were included in the Study Group and received an 8-day of Reflex Neurosensorimotor Integration (MNRI) treatment program.
The evaluative methods of effects of Reflex patterns treatment program included:
- The MNRI Reflex Assessment (Reflex Maturity Scaling) on each of the individuals in the study group before and after the rehabilitation program. This group received the MNRI Reflex sensory-motor neuromodulation rehabilitation program.
- Brain mapping QEEG records analysis (phases 1, 2, 3) done before and after rehabilitation with the MNRI.
Neurotransmitters Analysis (the test utilized the #9123 extended panel including 12 neurotransmitters, particularly, analysis of epinephrine, norepinephrine, DOPAC, dopamine, GABA, glutamate, serotonin, 5-HIAA, PEA, glycine, histamine and taurine and some other [1-3].
All three evaluation methods showed substantial positive changes in children with CP in corresponding areas after the MNRI treatment, particularly:
- 79.5% (62 out of 78) children displayed a reorganization of spontaneous brain electrical activity noted in an increase in brain frequency in range of alpha waves and a decrease in hi-beta activity in areas of the parietal and temporal cortex. This can be interpreted as a positive and stable regulatory effect of the MNRI program on neuromotor disorders causing deficits in the central nervous system. This may be connected with the positive and stable therapeutic effect of the MNRI method on motor disorders that originate in the central nervous system.
- The neurotransmitters analysis showed improvement in a) regulating effect, b) enhancement of stress regulation (taurine decrease); c) an antiinflammatory effect (improving cytokinesis), d) restoration tendency for nerve fibers (serotonin as a modulator for glutamate), d) hormonal changes supporting muscle tone regulation and motor control support.
- 66.7% (26 out of 30) reflex patterns showed significant improvement.
This study demonstrated that the neurodevelopment and overall functioning of individuals with neuro deficits, such as the CP, are not static in pathology and can be significantly improved with MNRI tools presenting a form of neuromodulation treatment.
Reflex parameters; Cerebral palsy; QEEG-Brain mapping; Brain waves; Neurotransmitters; MNRI-Masgutova Neurosensorimotor Reflex Integration Method
A reflex, as a unit and projection of the state of the Central Nervous System (CNS), has not been well studied in the field of the practical application of neuroscience. This study shows the results of the practical application of reflex patterns by specialists dealing with neurorehabilitation of individuals with diagnosed CP. Pathologies in the nervous system are reflected by dysfunctional and pathological reflexes, a topic that is not well understood and sometimes ignored by helping professionals. The concept of a reflex as a genetically given pattern and function of the brain, [4] is often difficult to understand. In infancy, it is active and becomes part of the overall functions of neurodevelopment and integrates automatically into the sensorymotor milestones in young children. It is also now understood to assist the CNS in managing everyday stress and life traumas. If a reflex is immature or dysfunctional then the effect of chronic stress and life trauma damages the CNS. The neuroscience of the problems and consequences of chronic stress and trauma damaging the CNS needs special attention and research to offer accurate information on best practices in neurodevelopment facilitation.
The MNRI (Masgutova Neurosensorimotor Reflex Integration) program was proposed by S. Masgutova, Ph.D. in 1989 in Russia [5]. This approach offers a novel solution on how to organize the treatment of reflex circuits leading them to proper neurophysiological functioning to balance the excitation-inhibition processes of the nervous system, Hypothalamus-Pituitary-Adrenal (HPA) stress-axis and resilience, and increased neuroplasticity.
The impact of sensory and motor modalities on the growth of nerve net-axons and dendrites, their myelination, and functions within overall work of the nervous system is investigated worldwide by many researchers [6-16]. Within the neurophysiological, pediatric, and psychological sciences, there is a range of programs addressing neuro deficits and developmental problems. These programs propose interdisciplinary approach including known forms of medical care (medication, surgery, orthotics, and many other), physical therapy, occupational therapy, speech-language pathology therapy, neurofeedback [17], dietary, and other motor-oriented therapies, such as Bobath Neuro-Developmental Techniques, V.Vojta Reflex Locomotion, Aqua-Therapy and various others [18-28].
MNRI research done earlier demonstrates evidence of the progress of the sensory-motor integration, improvement of motor milestones, and mobility of children with CP, also a positive effect on their immunity regulation in cases of bronchitis [7,19,29-33].
The MNRI® reflex integration program is based on the concept that dysfunctional and pathological reflex circuits and their components can be reconstructed or compensated by re-training of reflexes targeted at awakening the genetic sensorimotor memory in individuals. This type of neuro sensory-motor training results in significant neuro developmental changes that has been observed in increase of mobility, motor, and postural control, physical strength, and improvement in immune activity, as well as in emotional-behavioral, cognitive, and communication abilities [4,19,30,32,34-39].
Masgutova Neurosensorimotor Reflex Integration-MNRI is a noninvasive method used for evaluating and neurorehabilitation of individuals having neurological dysfunctions such as Cerebral Palsy (CP), Traumatic Brain and Acute Brain Injury (TBI and ABI), hyperactive Cerebral Palsy (CP), Acute and Traumatic Brain Injury (ABI and TBI), Autistic Spectrum Disorders (ASD), hyperactive disorders (ADD and ADHD), Fetal Alcohol Syndrome Disorder (FASD), and other diagnoses. Different clinical and objective brain studies have been carried out to evaluate the effect of the MNRI Method on the functioning of these clients. Long-term use of this method and research of its effect on the rehabilitation of children with neuro deficits has shown substantial improvements in different areas of their functioning: brain mapping, neurotransmitters, reflex patterns maturation, as well as clinical improvements in sensory-motor integration, postural and motor coordination, emotional-behavioral and cognitive processes [7,32].
The theoretical foundation for the MNRI approach comprises of ideas:
1) Sensory-motor integration is essential for neurological maturation and development in a human [10,12,15,40-42].
2) Motor development involves physical growth, movement coordination, postural control and differentiation with particular stages, and sensory-motor milestone dynamics for consciously learned abilities and skills [22,43-45].
3) The motor system, working at an automatic level, is based on integrated sensory reception/perception, processing of the sensory input by the whole nervous system and corresponding motor scheme/ response of a specific reflex circuit [10,13,42,46].
4) Within a healthy functioning reflex circuit its neurosensorimotor integrity and motor activity serve properly the survival mechanisms and create the foundation for physical and cognitive development [7,10,41,46-49].
The MNRI Program demonstrates the regulative effect on the above abilities of a child with CP through the concept and restoration of ‘stressed’ or ‘lost’ functions of the nervous system. It uses neuromodulation techniques that pave and reroute the nerve pathways of primary reflex patterns to bring them into a state of proper neurophysiological functioning which ultimately improves the balance of the excitation-inhibition processes of the nervous system, increases the HPA-stress resilience and neuroplasticity. The results of the presented study indicate that the neurodevelopment of individuals with CP-corrective and preventive-are enhanced with this unique novel form of MNRI neuromodulation treatment.
Earlier MNRI research, clinical studies, and reflex assessments of over 36,000 clients who underwent the MNRI® therapeutic programs demonstrate that this program reduces neurological deficits and facilitates neuromotor development. The therapy enables individuals diagnosed with the above disorders to reroute their early motor and reflexes patterns, motor coordination, a higher level of motor abilities and skills bettering their everyday functioning and learning [22,30,33,34,50].
This study found that the group of individuals with diagnosed CP was characterized with pathological sensory-motor reflex patterns correlating with pathologies in their brain maps (p<0.05). The longterm work of specialists using the MNRI method shows a significant positive effect on the normalization of the brain wave spectrum and neuroplasticity increase.
For this study, MNRI practitioners provided intensive MNRI Reflex Integration treatment for these children for 8 days (4 days of therapy sessions, one day off, and 4 days more of sessions) of 6 hours of intensive therapy daily with each client on a one-to-one basis. Each session was focused on a process of neuro correction called a reflex integration module, and particularly:
- MNR IR reflex Repatterning
- MNRI NeuroStructural Reflex Integration and Immune System
- MNRI NeuroTactile Integration
- MNRI Archetype Movements Integration
- MNRI Breathing Reflex Integration
- MNRI Stress and Trauma Release
- MNRI Proprioceptive-Cognitive Integration
- MNRI Oral-Motor/Visual-Auditory Reflexes Integration
Caregivers were given evening workshops and group lectures so they can continue the MNRI Home Program involving the abovelisted programs including theory and hands-on practice. The results demonstrated the significant effectiveness in improvement of the reflex patterns, QEEG, and neurotransmitters markers with the MNRI treatment program in an impressively short time of 8 days of MNRI sessions.
Cerebral palsy
Childhood Cerebral Palsy (CP, paralysis cerebral infantum), a Little’s neuromotor disorder accompanied by impairments in motor-postural programming, planning, and control resulting from permanent, nonprogressive brain damage in early stages of development-fetal or infancy [51-54]. Childhood cerebral palsy is a set of non-progressive central nervous system dysgenesis that can be resulting from damage to the brain during pregnancy (20%), perinatal stage (60%), or in the first years of life (20%) [43]. Causes of CP include chronic disease during pregnancy, malformations, fetal hypoxia, abdominal injuries to the mother, infection, and neuro-infections, perinatal trauma, prematurity, the influence of ionizing radiation, drugs or toxins including alcohol and cigarette smoking and consumption, hypoxia after birth, brain injury, and severe neonatal jaundice. The cerebral damage causes the upper motor neuron lesion that includes motor deficit, spasticity, impaired voluntary movement with the nonselective command, and motor discoordination. Dystonic and mixed cerebral palsy can also be caused by damage to the extrapyramidal tract [51]. Structural lesions in the brain are found in 80% of CP cases, most commonly within the white matter [55]. The corticospinal tract is responsible to maintain the spinal cord reflexes underlying the acquisition of motor skills [56,57].
It is known that the corticospinal tract activities shape the spinal cord motor function in a child from 0 to 3-year-old age [58]. Therefore, CP lesions of the corticospinal tract result in pathologies of spinal reflexes and motor impairments.
Depending on the area of damage of the CNS four main forms of the CP disease can be distinguished: spastic (pyramidal) and dyskinetic or extrapyramidal, and atactic (brain) and mixed forms [6,51-54,59,60].
Symptoms of CP condition
This study included children with diagnosed CP in the forms of 1) hemiparesis CP (spastic paresis of varying severity levels), 2) tetraplegia (spastic paresis of four limbs), 3) ataxia and hypotonia (mild cerebellar syndrome with mental disorders), and 4) dyskinesia, not including dystonia (extrapyramidal syndrome).
The main symptoms and developmental and postural/motor coordination issues of children with CP included:
- Muscle tone dysregulation, for example, being either excessively solid or excessively floppy
- Pathological muscles hypertonic and ‘misrepresented’ reflexes (spasticity), rigidity, muscle shortening
- Solid muscles with ordinary reflexes (inflexibility/rigidity)
- Abnormal muscle contractions causing involuntary writhing movements (athetosis)
- Absence of muscle coordination (ataxia)
- Tremors or pathological automatic responses
- Delays in achieving the sensory-motor and motor skills-pushing up on arms, sitting up independently or creeping
- Preference to use one side of the body, for example, using a single hand or dragging a leg while slithering
- Trouble standing (typical for CP to stand on toes), scissors-like stepping with knees crossing, wide walking or unbalanced steps
- Orthopedic deformities
- Drooling or trouble swallowing. The trouble with sucking or eating (biting and chewing)
- Delays in speech development and expressive language
- Trouble with precision in movements-gross and fine (getting and holding a pencil/spoon
- Seizures
- Issues with vision and auditory reception and perception
- Non-typical sensation for touch or pain
- Oral infections
- Psychological/mental conditions
- Urinary incontinence
- Others
The underlying issues investigated in this study included pathology and immaturity of reflex patterns in CP children. Reflexes activate the nervous system’s defensive mechanism triggering survival responses in situations where the hindbrain deals with reactions of survival, blocking the reasoning processes of the cortex. Rehabilitation of the reflex circuit supports the proper work of the whole nervous system and its defense mechanisms of children with CP [20]. Children with CP exhibit various dysfunctions in reflexes and reflex patterns, such as dysfunctions in sensory processing and motor/postural control, lack of balance, negative effects of hyper/hypo-protection, limitations in visual-auditory perception, as well as overall neurodevelopment and learning.
The MNRI method promotes the understanding that the natural course of development of a child equipped by nature with a complex system of natural resources, reflexes, and instincts “acts’ through reflexes and primary motor patterns organizing neural connectivity between sensory and motor axons, and their interneurons in the CNS, and developing body functions. The initial reflexes-reactions occur in children as automatic sensory-motor patterns. In this study, MNRI strategies were used to evaluate and provide exercises for reflex patterns correction to ensure neurosensorimotor development based on the clinical functioning, neurological status, and biological age of a child [61].
The MNRI neuromodulation methods used when working with children with CP puts forth the idea of bringing a proper model for ‘connectivity’ in pathological reflex patterns in a non-invasive way, not to activate their over-defensive responses, and offers a treatment modality that works in flow with genetically given static and dynamic reflexes of the brain-body system. This MNRI program uses the exercises as the “neuro-modeling“ or neuro-patterning the impaired reflex for training the brain to self-oriented move in. the direction of normal functioning [62-64].
The study is organized into our stages:
1) The first stage of research provided the Reflex Assessment, Brain Mapping-QEEG evaluation, and Neurotransmitter Analysis for the Study (n=78).
2) The second stage of the research provided the MNRI 8-day therapeutic sessions for participants in Study Group.
3) The third stage of research provided the post-tests of the same Reflex Assessment, Brain Mapping-QEEG evaluation, and Neurotransmitter Analysis for the Study group.
4) The fourth stage included the study data analysis and interpretation.
This study is a New England IRB approved study (#: 20160464, Legacy IRB#: 15-466, Action Date: 02/11/2016; A WIRB Copernicus Group Company), and also the Bialystok Medical University of Poland and The International Dr. Svetlana Masgutova Institute of Poland. All specialists leading the evaluations in the USA got certified by the NIH (National Institute of Health, Office of Extra Mutual Research) “Protecting Human Research Participants” in 2012-2017. Every participant was assigned codes to protect anonymity. Receipt of informed consent was received from all participants’ parents or legal guardians. MNRI assessments and treatments were administered by designated MNRI Core Specialists in the USA and Poland who successfully completed the requirements for Continuing Professional Education in MNRI and clinical hours (https://masgutovamethod.com/).
Demographic data
Seventy-eight children aged 2-19 years (M ± SD=7, 65 ± 4, 85) diagnosed with cerebral palsy were included in the Study Group, 18.9% (n=14) of them were female and 81.1% (n=60) male. The group consisted of 15 children aged 2-3; 23 age of 4-5; 16 children of age 6-9; 8 of them aged 10-11; 9-aged 14-15; and 7 children aged 16-19. Six subjects had diagnosed seizure and were taking medication and/ or supplementation at the time of QEEG tests and neurotransmitters sample submission. This study included children with diagnosed CP in the forms of: 1) hemiparesis (n=17), 2) tetraplegia (n=22), 3) ataxia and hypotonia (n=14), and 4) extrapyramidal dyskinesia (not including dystonia) (n=25). All Study Group individuals participated in the 8-day MNRI Reflex Integration program intervention.
Evaluation methods
MNRI reflex assessment: This assessment involves 30 reflex patterns (X1-X30) for all children in the study and control groups. The Study Group was given this assessment prior to MNRI treatment and after its completion of eight days at the treatment conference. This assessment of reflex patterns was based on the neurophysiological definition of unconditioned reflexes and their five parameters, such as: sensory-motor schema, direction of the motor response, muscle tone regulation/intensity, latency/timing of the response, and symmetry [23,30,34]. Parameters were evaluated based on these four features. For example, the first parameter of ‘sensory-motor schema’ (stimulus of a receptive field and motor response on biomechanical level) in the Robinson Hands Grasp Reflex checked the four features: 1) tactileproprioceptive stimulation on the upper palm to activate palm flexors and trigger a strong hand grasp response, 2) all fingers closure in a fist and thumb positioned above index and middle fingers, 3) extension of elbow in front of the body at 180°, and shoulder at 90° or more (in norm the differentiation between the palm, elbow and shoulder must take place with ability to shock absorption at joints), 4) arm/palms are hold horizontally away from the ground (abduction/adduction in the wrist joint are not present) [Table 1].
Scoring of fours features were done on a scale of 0-4, with “4” indicating full display of all four features in a parameter, and “0” indicating that normal responses were absent (all features were deviated from norm). The scoring of 5 parameters and their 4 features gives a maximum of 20 points meaning that a reflex pattern is maturity or integrated [Table 1]. The scores between 11 to 20 shows at functional development of a reflex pattern, and below 10 points (0-9)-mean that a reflex is on dysfunctional or abnormal level of development. The scores of 10 to 11.99 present a marginal level between functional and dysfunctional state of a reflex pattern of. Scores 16-17.99 represent the normal/functional level. The scoring system was validated by mathematical statistical research lead by Prof. A. Krefft of Medical University of Poland [22,35], and also the ANOVA test (IBM SPSS Statistics Grad Pack 22.00). The results were counted as statistically significant with p values (M ± SD) less than 0.001 and approached as significant, and not significant at p>0.05.
Reflex patterns were further grouped for deeper analysis of motor-postural regulation according to body motor planes, with ten reflex patterns in each, and particularly, to: sagittal (medial-lateral), horizontal (superior-inferior), and vertical (anterior-posterior) planes [23,30,34].
Brain mapping-QEEG: Brain Mapping tools used in this study have been: the Deymed Truscan 32 (Deymed Diagnostic, Payette, ID) EEG equipment combined with Neuroguide QEEG (Applied Neuroscience, Inc.) software. QEEG analysis was completed using the Neuroguide software (Applied Neuroscience, Inc.) targeted at recording of 19 channel digital EEG (Deymed Diagnostic, Payette, ID). Approximately 1-3 minutes of artifact-free eyes closed or eyes open EEG segments were selected after previously recording EEG with the Deymed, Truscan 32, (Deymed Diagnostic, Payette, ID) and taken to further QEEG analysis. For evaluation of the brain wave spectrum of children with CP, two EEG sessions before and after the 8-day-MNRI program of six hours day interventions were done. Spontaneous EEG activity was recorded in a 10-20 electrode system using 16 leads. The EEG recording underwent an elimination of artifacts, after which the software produced QEEG maps of the EEG frequencies. The Fast Fourier Transform (FFT) algorithms were used in computer analysis. Spectral maps were compared in relative and absolute scale for the measured signals obtained before and after MNRI treatment. The analyzed the EEG spectrum from 0.0 Hz to 30Hz, divided into five sub-ranges: 0.5-4.0 Hz (delta), 4.0-8.0 Hz (theta), 8.0-12.0 Hz (alpha), 12.0-20 Hz (beta) and 20-30 Hz (high beta).
Below the example of the QEEG analysis of the child with the CP using the NeuroGuide (Applied NeuroScience Inc.) software and previously recorded on Deymed EEG is performed.
For understanding of the results of the brain wave spectrum analysis the main description of its waves was used:
Gamma waves: These are involved in processing of consciously/ cortically oriented tasks: high cognitive functioning-deep focusing, selective perception, analysis, synthesizing, critical thinking and learning, memorizing of complex matters, intense information processing. The 30-40 Hz gamma wave is essential for the connecting of our senses (perception). This brain wave corresponds to the states of modulating perception and consciousness, ‘higher virtues’ altruism, high enthusiasm, and the ‘universal love’.
Individuals with mental and learning disabilities tend to have lower level of gamma brain wave activity than neurotypical individuals [32,65-67]:
- Frequency range: 30 Hz to 100 Hz (Highest)
- Too much of gamma waves result in: anxiety, high arousal, stress
- Too little of gamma waves result in: ADHD, depression, learning disabilities
- Optimal of gamma waves helps: binding senses, cognition, information processing, learning, perception, REM sleep
Beta waves: High frequency low amplitude brain waves have a stimulating effect and are observed while we are in awake state and in full consciousness. They are active while logical and critical thinking, focusing, writing, reading, socialization takes place, and when complete school or work-based tasks must be done. Caffeine and other stimulants increase the beta activity. Too many high-beta with its higher frequencies are corresponding to intense arousal, excessive stress, and anxiety
- Frequency range: 12 Hz to 40 Hz (High)
- Too much: High arousal, anxiety, worries, high level of adrenaline, inability to relax, tendency to get stressed easily
- Too little: ADHD, difficulties with cognition, daydreaming, depression
- Optimal: Conscious selective perception, easy focusing, productive memory and problem solving
Alpha waves: Alpha is the frequency range taking place between beta and theta-waves; it links the conscious thinking/control and subconscious mind processes, helps to calm down and deep relaxation. These waves are dominant during quiet thinking, in meditative states. Alpha is ‘the power of being in “the present”, “here and now’. Alpha wave is a resourceful state for brain rest and restoration. Alpha aid overall mental processes coordination, calmness-alertness balance, mind/body integration and spontaneous learning.
At times when we become stressed, a so-called “alpha blocking” phenomenon may occur which triggers hi-beta activity dominating overall alpha resulting in becoming too aroused. In these cases the beta waves “block” out the production of alpha, this is why we can become hyperactive and “lose inner peace”.
- Frequency range: 8 Hz to 12 Hz (Moderate)
- Too much: Inability to perceive clearly and focus, daydreaming, too relaxed
- Too little: Tendency to high stress, anxiety, insomnia, and OCD
- Optimal: Inner peace, comfort, relaxation
Theta waves: Theta waves are involved in “deep and raw” emotions, involved in daydreaming and sleep. Theta is our door to memory, learning and intuition. In theta, we can concentrate on our internal perception focused on signals coming from our sense modalities. This wave allows withdrawing ourselves from the external world. It is that “twilight state” which usually can be experienced at awake state or when we drift off to sleep. In theta we can dream with pictures que imagery, information processing moment, and intuition beyond our normal awareness and conscious control. It’s where we hold our ‘stuff ’, troubled history, our fears, and also nightmares. It is a helpful wave also for restorative sleep. Too much dominating of theta activity may make people predisposed to depression, and may cause “too deep” relaxation, and semi-hypnotic. Theta helps to improve calm processing, creativity, intuition, and makes us feel more spontaneous and natural.
- Frequency range: 8 Hz to 12 Hz (Moderate)
- Too much: inattentiveness, hyperactivity, impulsivity, ADHD, depression
- Too little: Stress, poor emotional awareness, anxiety, worries
- Optimal: Relaxation, creativity, intuition, emotional connection
Delta waves: The slowest waves recorded in a human brain. Found most often in infants and young children. With our development we tend to produce more beta and less delta waves even during our deep sleep stages. These waves are associated with the deepest levels of our unconsciousness, such as deep relaxation and restorative, healing sleep. They also are involved in sub-and unconscious functions of our organism: regulating heart rate, breathing and digestion. Production of delta waves are involved in feeling completely rejuvenated after the wake up from a deep, restorative night’s sleep. Delta waves are the source of empathy. Abnormal delta activity may cause learning disabilities or difficulties in maintaining conscious awareness (like in cases of brain injuries).
- Frequency range: 0 Hz to 4 Hz (Slowest)
- Too much: inability to think, learning problems, severe ADHD, in cases of brain injuries
- Too little: Inability to revitalize the brain, inability to rejuvenate body, poor sleep pattern
- Optimal: Supporting natural healing, restorative/deep sleep, and immunity [Figure 1].
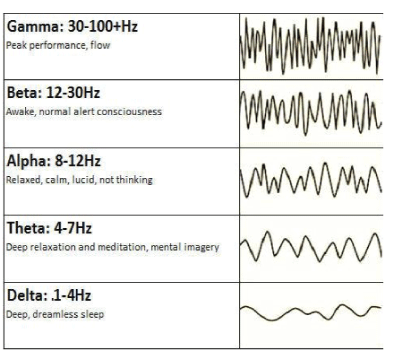
Figure 1: Brain wave spectrum: types and frequency bands.
Directions of analysis included
Quantitative analysis: The EEG was recorded from multiple locations across the scalp, digitally converted and analyzed and then presented in a format providing colored head maps to indicate levels of deviation from mean reference scores. The recording was done relative to Brodmann fields and characteristics of brain waves affecting different areas of life and activity: the cognitive, physical-motor, emotional and behavioral in participants of the study group. The brain mapping main markers-dimensions for the analysis were: Magnitude, Dominant Frequency, Interhemispheric Connectivity, Coherence, and Hemispheric Asymmetry. The mean reference scores were derived from analysis of: a) normative and non-normative neurometric databases and b) significance levels are based on clinical correlations with psychometric instruments.
Qualitative analysis included the comparative analysis of pre and post-evaluation of maps in perspective of the above QEEG map markers. The analysis of the markers was chosen based on clinical relevance and correlation with psychometric and neurocognitive measures. Also, the comparison with data on changes in reflex patterns and neurotransmitters were done.
a) Quantitative analysis of the QEEG is based on the knowledge of neurophysiology and the functions of particular brain regions. Identifying changes localized by the QEEG in the context of behavioral, sensory or communication symptoms can help to better understand how the MNRI therapy affects neurosensory brain activity. Higher brain structures evolved in their development from lower levels, therefore the effect of cortical centers depends on the proper functioning of subcortical structures. This study developed the QEEG analysis in accordance with the scientifically known and accepted Brodmann fields.
The present research uses the Electroencephalogram (EEG) method for recording and analysis of bioelectrical brain function from the surface electrodes located on the skull, and creating the EEG image of the bioelectric activity pattern emerging in the cerebral cortex. The QEEG allowed the recording of signals originating from neurons with intense bioelectrical impulses or functional potentials following from postsynaptic areas (PSP-postsynaptic potentials) and longterm neuronal depolarization [42] created as a result of synchronous stimulation of nerve cells. The amplitude of the EEG signal is usually from 20μV to 100μV. A record with amplitude below 20μV is defined as low voltage, and above 100μV as high voltage. For the evaluation of EEG spectral values and quantitative QEEG analysis, frequencies in the range of 0 to 100 Hz are used, and clinical practice is usually recorded in the range 1-70 Hz [68]. Automatic caps have been used to record EEG signals, which ensure high repeatability of electrode location, convenience and easy assembly (=gathering, bringing together), which is important in clients, for example, with impaired sensory sensation, like with CP. The measuring electrodes are located in accordance with the recommendations of the International Federation of Clinical Neurophysiology IFCN-in the 10-20 system. Built-in electrodes are made of non-reactive metals, mainly silver or silver coated with silver chloride, sometimes also with gold and platinum. The purpose of the electrodes is to replace the ionic currents flowing on the surface of the head with the electronic current and send this current to the amplifying apparatus [44].
During the test, brain wave activities with ranges presented in table 2 were recorded [32,67,66]. [Table 3].
Table 2: Characteristics of brain waves.
Table 3: Areas of the brain and electrodes recording electrical stimulation.
b) Qualitative analysis included the comparative pre and postevaluation of maps within the following dimensions: Magnitudeaverage of the amplitude of the EEG signal, measured peak (+) to peak (-) across the time sampled.
Absolute Power means the level of activity within a given frequency band of brain waves. The activity in each frequency band is demonstrated in the examples below in order of slowest (left)- Delta to fastest (right) Beta.
Activity in each frequency band is compared to a normative database to screen the presence of any possible deviation from the norm. The results for each frequency band were presented with the topographic activity maps in green color as an average activity, and red as a large increase in activity, yellow color moderate increase in activity compared to the norm, and blue as a large decrease [Figure 2].
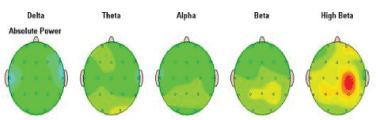
Figure 2: Absolute Power: amount of activity within a specific frequency band of brain waves in order of slowest (left)-Delta to fastest (right) Beta.
Dominant Frequency-usually in alpha range-EEG frequency predominant in the occipital region dominating during the awake state (recorded in eyes closed condition).
Relative Power is interpreted as the relative level/amount of activity within a specific frequency band compared to all the other frequency bands. Relative activity in each frequency band in our study participants was compared to the norm to find out the presence of any abnormality. The results for each frequency band were demonstrated with the topographic activity maps. In the example below, green is representing average activity, red-a large increase in activity, yellow-moderate increase of activity, and blue a large decrease [Figure 3].
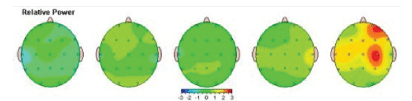
Figure 3: Absolute Power: amount of activity within a specific frequency band of brain waves in order of slowest (left)-Delta to fastest (right) Beta.
Interhemispheric Connectivity degree of connectivity between right and left hemispheres of the brain (usually based on comparison to the database).
Coherence refers to the similarity in EEG waves over different areas of the brain, i.e., the timing of activity in one area compared to another. Coherence marker in each frequency band was compared to the norm to screen presence of any suspected deviation. The results for each frequency band are given on a diagram with the topographic connection maps. Thick lines represent greater abnormalities vs. the norm; red refers to increased coherence, and blue-to decreased coherence [Figure 4].
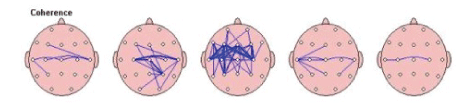
Figure 4: Coherence: the similarity in EEG waves over different areas of the brain (i.e., the timing of activity in one area compared to another); in order of slowest (left)-Delta to fastest (right) Beta.
Interhemispheric Asymmetry. Asymmetry shows a relationship between the amounts of activity in one brain area compared to another. Inter-hemispheric marker allows to measure differences between each side of the brain, while intra-hemispheric-between different areas on the same hemisphere.
Asymmetry in each frequency band was in our research compared to the norm database to screen the presence of suspected deviations. The topographic connection map sheres how the thick lines demonstrate greater deviations from “norm” red representing increased asymmetry, and blue-decreased asymmetry [Figure 5].
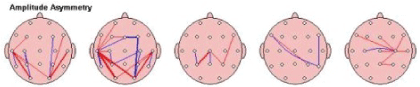
Figure 5: Asymmetry: the relationship between the amounts of activity in one area of the brain compared to another; in order of slowest (left)- Delta to fastest (right) Beta.
These dimensions-markers were chosen based on clinical relevance and existing correlation with psychometric and neurocognitive measures in the QEEG method.
Phase lag is the marker measuring the functional connectivity. The Phase Lag Index (PLI) allowed measuring the asymmetry of the distribution of phase differences between two signals in brain maps of study participants with CP.
Neurotransmitter analysis
Neurotransmitters are the body’s molecules used by the nervous system as endogenous chemical messengers to transmit boost, and balance signal messages between neurons and from neurons to muscles or glands. Interaction of two neurons (i.e., between sensory and motor, or sensory and interneuron, or motor neuron and muscle/ gland cell) happens in the synaptic cleft (gap) between the neurons. In synapse, the electrical signals following via the axon are briefly converted into chemical molecules or neurotransmitters causing an ext step of specific electrical response in the receiving neuron [69,70].
These chemical neuro-messengers can influence a wide variety of physical and also psychological functions including heart rate, sleep, appetite, mood, emotions, and fear. Billions of neurotransmitter molecules function constantly to support our brains to manage breathing, heartbeat, learning and focusing.
A dozen small-molecules known as neurotransmitters and over 100 different neuropeptides in our organisms can be categorised into three main types: acetylcholine neurotransmitter (1), biogenic amine molecules (2), and amino acids (3).They interact via signals targeting specific neurons. A neuron is influenced by a neurotransmitter in three ways: in excitatory (A), inhibitory (B) or modulatory (C). An excitatory transmitter activates the action potential or electrical signal in the receiving neuron, while an inhibitory transmitter prevents this signal. The excitatory/inhibitory function of a neurotransmitter depends on the receptor it binds to [69].
Neuromodulators are designed genetically to display different functions as they activate larger numbers of neurons at the same time, and as they are not restricted to the synaptic cleft between two neurons. This ability of neuromodulators allows them to regulate the work of neurons operating over a slower time course, than excitatory and inhibitory transmitters [69,70].
These chemicals and their interactions are involved in all functions of the nervous system as well as controlling functions of the physical body. For instance, GABA synapses considered typically to be inhibitory, however being inhibitory, they also are excitatory, with coexist is regulated in the cerebellar interneuron network. Psychoneuroimmunology studies have shown that the CNS has highly complex interactions with the endocrine system and immunity. Although the mechanisms of interactions of GABA inhibitory and excitatory are not fully discovered, it is known that stress and trauma can induce modulation of the immunity mechanisms, acting via the HPA (hypothalamic-pituitary-adrenal) stress-circuit and the Sympathetic-adrenal Medullary (SAM) axes [47,71-73].This study aims attesting and analysis of such neurotransmitters in children with CP, as: epinephrine (1), norepinephrine (2), DOPAC (3), dopamine(4), GABA (5), glutamate (6), serotonin (7), 5-HIAA (8), PEA (9), glycine (10), histamine (11), and taurine (12), and some other.
Below a brief overview of the transmitters used in our study is presented:
Gamma Amino Butyric Acid (GABA) is a calming, major inhibiting transmitter in a human organism. Its role is to decrease the neuronal activity and to increase “the calming effect” by restricting neuroelectric activity including reducing anxiety [74]. The activity of GABA increases during sleep time. It is responsible for setting the circadian rhythms.
In children with CP reduced GABA causes improper functional and anatomic connectivity at the motor cortex (cortical layers III, IV, and VI), and particularly thalamocortical and corticocortical connectivity, which can be an ‘attempt’ of brain to adapt to brain injury condition that has adverse effects on the development of motor plasticity [75]. This improper working mechanism leads to deficits in coordinating the sensory modalities processing and complex motor skills, which depends on plasticity in the motor cortex. The decreased GABA in the brain stem in CP clients causes the spastic syndrome [76]. Thus, increase of GABA transmitter in the motor cortex plays an important role for improvement of control over the motor abilities.
Glutamate as an excitatory neurotransmitter enhances signal transduction between axons; its functions are related to learning and memory. It is one of the most common neurotransmitters as the amount of glutamate in the brain is larger than any other amino acid. Tissue extracted from the brain contains 5-15 mmol glutamate per kg. Being an amino acid, the glutamate impacts not only the cognitive and learning functions, but also metabolic function and ammonia detoxification. In a child with CP the glutamate (excitatory amino acid) levels increase, while inhibitory amino acid levels (GABA) decrease; this imbalance may contribute to the pathogenesis of CP [77]. Too much glutamate damages the delicate immature oligodendrocytes and neurons, leading to injury in white matter [78].
Norepinephrine is a sympathetic nervous system catecholamine triggering the body to move into a “high alert” state at a moment of real or imagined danger and is responsible for increasing heart rate and blood pressure. Norepinephrine serves to activate not only pre-but also postsynaptic adrenergic receptors. Norepinephrine is released in the brain as the locus coeruleus is stimulated and action potential occurs. It is also important for long-term memory by influencing surges in specific neural circuits. When norepinephrine binds to a receptor and stimulates a nerve to respond, it functions as a neurotransmitter. In stress caused by sudden/unexpected touch, sound, visual stimulus, or loss of stable body position in space, this neurotransmitter activates the fight-or-flight response resulting in immature and reactive Moro and/or Fear Paralysis response, called ‘adrenalin rush’, which is chronic response in children with CP. Noradrenaline is produced in the locus coeruleus located in the pons of the brainstem, a sympathetic ganglia located near the spinal cord or in the abdomen, and the adrenal glands [19,79,80].
Epinephrine (adrenalin) is another catecholamine functioning as a flight/fight neurotransmitter-hormone released when the body is under extreme stress. It is closely related to norepinephrine, synthesized from norepinephrine in the adrenal medulla. Norepinephrine and epinephrine stimulate beta and alpha receptors, the difference is that norepinephrine primarily affects the alpha receptors, whereas epinephrine activates all three beta receptors as well as alpha receptors. In stress caused by sudden/unexpected touch, sound, visual stimulus, or loss of stable body position in space, this neurotransmitter activates the fight-or-flight response resulting in reactive Moro and/or Fear Paralysis responses, which is improper and a chronic response in children with CP [81].
Dopamine is the third excitatory neurotransmitter produced in two areas of the brain: midbrain, and the basal ganglia (substantia nigra) playing an important role in reward (ventral segmental), learning and movement. High levels of neuromelanin in dopaminergic neurons cause substantia nigra to appear darker than surrounding areas. One function of dopamine is movement initiation and motor control, and the caudate and putamen with the striatum in the basal ganglia are connected through a bundle of dopamine neurons that forms the nigrostriatal pathway considered a facilitator of movement. Dysfunction in dopamine metabolism in children with CP results in deficit of motor programming, planning and control. The Dopamine (DA) transmits via areas of the brain associated with motor learning (e.g. DRD1, DRD2, DRD3, COMT, DAT) and/or to activity-dependent brain plasticity (e.g. BDNF) which is decreased in children with CP, and affects their motor learning and cognitive processing [82]. The decrease of dopamine transmission influences the motor skill, which becomes insufficient; this is why the dopaminergic medications (like levodopa) or interventions are used in neurorehabilitation to gain better results in motor responses.
Serotonin (produced in the enteric nervous system of gastrointestinal tract and in the central nervous system) displays a range of important functions in overall health and wellbeing. This nerve cells chemical serves as one of the modulators for voltage potential regulating glutamate excitation. Serotonin is a metabolite of 5htp; produced up to 90% in the gastrointestinal tract, although it is also found in blood platelets and throughout the entire central nervous system. Serotonin is involved in regulation of smooth muscle contraction, cyclic body process, and nerve impulse transmission. It elevated levels in the spinal cord can be a cause for pathophysiological mechanism of hypertonia-pathological increase of muscle tone in CP [83].
Taurine is involved in the survival of neurons following a synapse, known as “the trophic factor”. As a neuromodulator it regulates the level of calcium in our organisms. Taurine is a protectant against glutamate-induced excitotoxicity by limiting increase of intracellular calcium level, achieved by shifting the ratio of Bcl-2 and Bad ratio in favor of cell survival and reducing the endoplasmic reticulum stress. Taurine plays an integral part in control of the amount of sensory information following to the cortex. Taurine plays a certain role in helping the brain to turn off activating inhibition processes at night by activating GABA inhibitory leading to deep sleep. The body cells in a child with CP are lacking taurine, which is associated with major pathologies and poor health. Taurine plays a neuro-corrective role in treatment of diseases of muscles, the central nervous system, the cardiovascular system, mitochondrial encephalopathy, metabolic disorders. Taurine’s main functions are: antioxidation control, energy metabolism regulation, gene expression, stress, neuromodulation, and calcium homeostasis [84].
Histamine fulfils various physiological bodily functions, one of which is the increase of the excitability of the CNS and regulation of “whole brain” activity. The hypothalamus ventral preoptic center assists in turning off histaminergic tuberomammillary cells which are linked to the sleep/wake cycle resulting in diurnal changes in brain function. Many functions of the hypothalamus are controlled by histamine including vasopressin that is physiologically regulated by histaminergic neurons.
Glycine is an inhibitory controlling and inflammation regulating neurotransmitter playing an important role in overall health and acting preventatively towards the body wholeness and health. It is found in the CNS, specifically in the spinal cord, brainstem, and retina. Being involved in manufacturing of immune-related hormones, it also participates in immunity formation. Glycine is produced in the liver in limited amounts and is used by organisms for detoxification. Glycine supports the synthesis of bile acid, amino acids, and takes part in the building of DNA, RNA and protein. As a precursor to proteins glycine is found in collagen, which contains about 35% glycine. Collagen, in its turn, is a major component of muscle tissue impacting its strength and tone. Thus, glycine participates in the development of collagen’s helix structure in link with hydroxyproline (amino acid for fibrillar collagens comprising approximately 13.5% of the protein) for muscles, all connective tissues and bones [85].
CP is a condition associated with improper muscle tone regulation and osteoporosis (or decreased bone density) resulting in fractures with minimal or more severe injuries that impair everyday postural and sensory-motor functioning. Particularly, the cartilage in children with CP tends to transform into bone and continues throughout their lives. Emerged deficit in the collagen fibers causes the deposition of calcium salts. Proper production of glycine can prevent over-firing the muscles (spasticity), osteoporosis, and risk of bone fractures in children with CP [86]. The impact of the MNRI exercises on glycine can support the anatomy and structure of muscles mass, bones and their metabolism.
PEA (Phenylethylamine) is an excitatory neurotransmitter participating in modulation of action potential to enhance glutamate activity and transmitter firing, and acts as a CNS stimulant.
PEA has a significant impact on body alertness, activation of the HPA stress-axis, and physical performance and on physiological aspects of mood. Emotions are controlled by the limbic system due to a very high concentration of PEA impacting motivation, emotions and socialization. Because it is an endogenous stimulant, PEA enhances dopamine, norepinephrine and serotonin. PEA causes fast impulsemediated release of catecholamines (dopamine and epinephrine) and serotonin in the brain, leading to rapid improvement of cognitive functions, increase of attention, awareness, and ability for pleasure, mood, libido, and overall sense of wellbeing. Low level of PEA in children with CP leads to hyperactive syndrome [87]. This neurotransmitter helps to fight against certain pathogenic strains [88]. Low or high concentrations of PEA may be associated with specific psychological disorders (e.g. attention deficit hyperactivity disorder, depression) [88]. Children with CP have a tendency for a low concentration of PEA in their brains, the good news is that these PEA brain levels can be increased.
All the urine samples were processed by the Neuroscience Lab (WI) and Mr. Brandon Peacock, and neither had a vested interest in MNRI. Independent scientists analyzed the results and compiled statistics. These scientists were not given any details regarding the treatment or suspected outcomes.
MNRI treatment and intervention programs used at the conference for individuals with CP: The MNRI Treatment and Intervention Program of Reflex Neuromodulation and Integration was used for individuals with diagnosed CP similar to the way all other research had been carried out in the MNRI studies [22,23,32,33], and included, particularly, the next sub-programs:
Reflex Repatterning-focuses on improving the connectivity between the sensory and motor neurons activity in a reflex circuit, and paving its pathways that can be seen in improvement of motor programming, planning and control, sensory-motor milestones, and also motorcognitive skills [19,28,89,90].
Neuro Structural Reflex and Immune System Integration-aims at improving the functions of reflex patterns responsible for muscular tone regulation in the trunk, abdomen, and neck and limbs, postural control, spine strength and flexibility, release of negative effect of protective tendon guard and creating positive protection and the feeling of being secure; for regulation of the immune system functions, creating the immunomodulatory effect aimed at improvement of functions of the T-1 immunity, cytokinesis, CD-4, CD-8 and other immune cells functions, anti-inflammatory effect, regulation of immunoglobulins (IgE, IgG and other) [50,91,92].
NeuroTactile Integration-focuses on the regulation and normalization of tactile receptors sensitivity level (hyper- or hypo-), coordination of receptors links, skin dermatomes, receptive zones of reflex patterns and overall peripheral and central nervous system for support of reflex repatterning and integration [90].
Archetype Movements Integration-targets at the improvement of primary biomechanics of sensory-motor patterns (stimulus+extension, flexion, rotation, stretching-compression, and other) giving support for structural aspect of numerous reflex patterns, development of automatic and consciously learned motor abilities and skills; also postural and motor control, including improvements in speed of perception, focusing, and memory, in sensory-motor integration, and cognitive functions [28,93].
Breathing Reflex Integration-aims at the regulation and normalization of breathing reflexes, and the increase of residual volume of the lungs for normal and easy breathing and creating sufficient protection and survival [30].
Stress and Traumatic Stress Release- focuses on those reflex patterns that impact the HPA stress-axis for letting go past negative stress and trauma and feeling of pain, for normalization of level of stress hormones and optimizing the neurotransmitters regulation [19,89].
Proprioceptive-Cognitive Integration-is oriented at the goal of improving proprioceptive-vestibular system and equilibrium-related reflexes (gravity, balance, grounding, stability, mature gait) for support of postural and motor control, with improvement also with motorcognitive hands-on-tasks.
Oral-Motor/Visual-Auditory Reflexes Integration-deals with tasks oriented at improving the oral-motor functions (Babkin, chewing swallowing, coordination with breathing), articulation and speech abilities, as well as visual (pursuit eye tracking, saccadic eye movements, ocular-vestibular, optokinetic) and auditory (stapedial, Asymmetrical Tonic Neck) reflex patterns [94].
Results and discussions of the obtained data on the effect of the MNRI Reflex Integration Program on improvement in: A) reflex functionality (level of development), B) brain wave spectrum, and C) neurotransmitters in children with CP demonstrate novelty and unique possibilities of the perspective neurorehabilitation work for children with CP are presented below:
Reflex assessment results in children with CP [Table 4]
Table 4: Reflex Profile of individuals with brain dysfunctions results before and after the MNRI Program (in 4 days on, one day off and 4 more days) 9 days total.
The comparative analysis of the MNRI Reflex Assessment results in pre-and post-tests demonstrated the following important findings:
First, the level of development of reflex patterns in the Study Group of children with CP (n=78) was overall immature and dysfunctional, particularly:
- 60%/18 patterns (out of 30) were on a Severely Dysfunctional level (4-5.99 points),
- 33.33%/11 patterns-Dysfunctional (6-7.99 points),
- 3.33%/1 pattern-Light Dysfunctional (8-9.99 points), and
- 3.33%/1 pattern-Marginal between Dysfunctional and Functional level (10-11.99).This shows that fact that the Profile of Reflex Development in these CP children is deeply dysfunctional though depending evidently on the severity of their brain damage.
Second, the results of post-assessment of reflexes of these CP children after the MNRI treatment show that 66.7%/26 of their reflex patterns (out of 30 patterns) have changed within 8 days of the MNRI Therapy Program and, particularly:
- Only 6,7%/2 patterns (out of 30) were on a Severely Dysfunctional level (4-5.99 points),
- 27%/8 patterns-Dysfunctional (6-7.99 points),
- 63.33%/19 pattern- Light Dysfunctional (8-9.99 points),
- 3.33%/1 pattern-Functional but very Low level (10-11.99) [Figure 6].
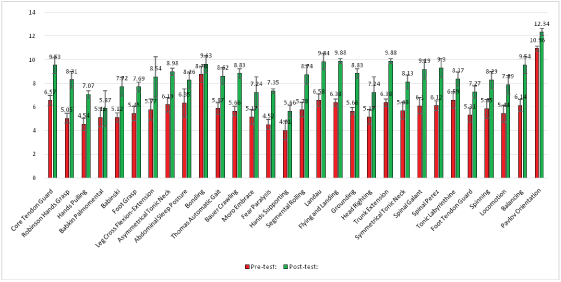
Figure 6: Reflex development level in individuals with cerebral palsy diagnosis (n=78) before and after the MNRI® program (in 8 days).
Third, results of the post-assessment demonstrated significant improvement in reflex patterns still indicated that they were at a dysfunctional level and needed further regular corrective work.
The results of pre-and post-Reflex Assessment demonstrate that dysfunctional or pathological reflexes have a. chance to be changed with specially oriented manual neuromodulation treatment vs. traditional concepts approaching the abnormality as a non-changeable, static state.
Our clinic experience shows the need of 2-3 years of regular work in the Program to better the functions of reflex patterns to move them to a functional level and, next, to improve their sensory-motor integration and milestones [Figure 7].
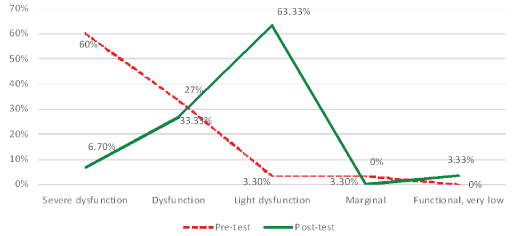
Figure 7: Reflex patterns in children with cp (n=78) before and after the MNRI® treatment program.
The MNRI neuromodulation program effect on brain maps changes in children diagnosed with CP
Three individual EEG recordings were carried out: phase 1-prior to the MNRI Conference, phase 2-prior to rehabilitation exercises and after (day nine) as a final measurement [23,32] and phase 3-after the end of the of MNRI rehabilitation program on the day 9.
Pre-evaluation results:
a) The overall analysis of the ‘normalcy’/deviation of the brain wave spectrum of children diagnosed with the CP demonstrated the fact of wave spectrum disorganization in 83, 33% (n=65 out of 78) of them identified as having QEEG abnormalities, 48, 72% (n=38) out of which presented idiopathic seizure type of brain activity, and 10.26% subjects (n=8) with diagnosed seizures prior the research.
b) A qualitative analysis of EEG records from phases 1, 2, and 3showson certain typical tendencies and differences in maps of these children before MNRI therapy, and particularly:
The most common deviations from norm identified by brain mapping were over expression of delta or theta power in the frontal or temporal areas of the brain.
An elevated frontal or occipital beta or high beta power were also frequently recorded. The results in an absolute scale demonstrate the tendency for dominance of low frequencies in the upper frontal leads, and the lowest amplitude in the vicinity of the temporo-occipital leads, which is coherent to the results of other authors studied the effects of the MNRI on brain marring [23,32].
Theta and some delta activity are located mainly in the occipital region in contrast to proper alpha activity [23].
The analysis of the brain wave in the fronto-parietal leads showed that the fast frequencies from beta waves are of the highest amplitude in the brain areas. This data means that children with CP in the study group had obvious issues with the quality of focusing, presence, and awareness. Some minor periodic voltage fluctuations in delta and theta frequencies took place with no significant changes throughout all three phases.
The analysis further demonstrated a mixture of slow delta-theta waves mainly taking place in the frontal and parietal leads, while the alpha waves were mainly observed above the occipital leads.
Post-evaluation results (after 8 days of work on the MNRI Program)
Overall degree of brain maps improvement in children with CP: All post-brain map results after MNRI therapy showed that 79.5% (n=62 out of 78) study participants demonstrated positive changes in previously identified QEEG abnormalities. The levels of improvement after the MNRI intervention varied from subject to subject and only a small number of participants didn’t show significant brain mapping improvement 8.97% (n=7). However, they demonstrated a tendency for improvement of range of abilities in the physical-motor area, of focusing, language and in sensory-motor integration processes.
Improvement in specific brain areas after the MNRI Program:
a) The analysis of the brain maps of children with the CP before and after the MNRI program shows the tendency for normalization of the QEEG records, and particularly, in the frontal, central, temporal and occipital areas. The findings of comparative analysis show statistically significant improvement of the brain maps and, particularly, in: Frontal Eye Fields (FZ-Area 8), the Left and Right Frontal Lobe Area-Dorsolateral Prefrontal Cortex (F3 and F4-Area 46), Post central Gyrus (C3 1, /C4-Areas 2, 3), Orbital, temporal and lateral frontal brain region (F7-F8-Areas 10, 11, 47); Angular Gyruspart of Wernicke’s area (P4-Area 39), Primary Auditory Association Cortex (T3/T4-Areas 41, 42), Posterior-temporal Region (T5-T6), Somatosensory Association Cortex (Cz-Area 5), the Occipital brain region (O1-O2)(See areas on the picture with electrodes), which could improve the corresponding brain functions [Figure 8].
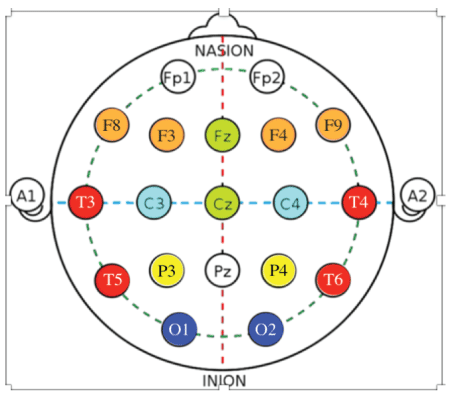
Figure 8: Brain wave spectrum profile of dynamic changes in specific bands after the MNRI Reflex Integration Program.
b) Analysis of the potentials in the fronto-parietal leads showed that fast frequencies from beta waves were found at the highest amplitude. This data leads to the presupposition that the quality of focusing, awareness and control in the Study Group had increased on a significant level.
The more detailed data on maps analysis shows the marked increase of global delta, beta, and high beta powers as well as an increase in frontal/temporal theta and alpha powers took place meaning that the abnormalities in hyper/hypo-coherence and phase lag improved similar to those of the previous study done with MNRI [32]. Some results of this analysis showing the statistical significance (*blue color) in the form of graphs and their interpretations are presented.
Electrodes activity recording from frontal eye/visual fields: FZArea 8
Findings: Significant changes in electrode position in FZ (Area 8) in the 10-20 system located in the brain area of Dorsolateral Prefrontal Cortex in the Study Group after the MNRI Program compared to the pre-test results took place [Figure 9].
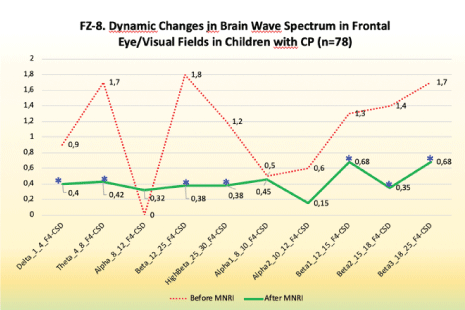
Figure 9: FZ-8. Dynamic changes in brain wave spectrum in frontal eye/visual fields in children with CP (n=78).
Improvements in:
- Hand-eye coordination, space-time orientation
- Visual-auditory coordination
- Visual-oral and fine motor imitation
- Comprehension, ability to follow instructions
- Precision of physical movements
- Focused perception and better orientation/recognition of gestures
- Ocular-vestibular control
Electrodes activity recording from right frontal lobe areadorsolateral prefrontal cortex: f3-f4-area 46
Findings: Significant changes in electrode position of F3 and F4 (Area 46-Dorsolateral Prefrontal Cortex), which in the 10-20 system is located in the area responsible for executive functions: motor planning, receptive language, conscious emotional control, behavioral decisions, after the MNRI Program compared to the pre-tests showed certain progress [Figure 10].
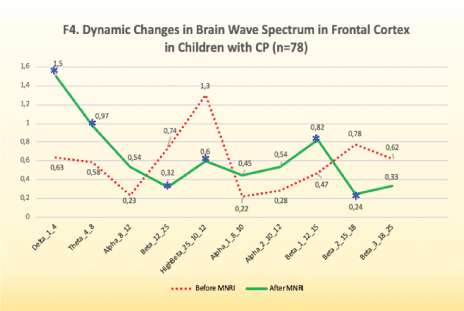
Figure 10: F4. Dynamic changes in brain wave spectrum in frontal cortex in children with CP (n=78).
Improvements are noted in the following abilities:
- Comprehension, ability to follow motor instructions
- Motor planning and control
- Space-time orientation for gross, medium and fine motor coordination
- Perception and processing of oral information and better orientation in direct and indirect meaning of content of stories/texts
- Desire and attempt for interaction and expressive language; more variable voice modulation
- Regulation of motor aspect of emotional expression, less reactivity
- Variability of gestures
- More freedom of the fine motor coordination from whole body response involvement
- Facial expression control
- Reasoning of own and other people’s behavior
- Reflex patterns functions, particularly for Symmetrical Tonic Neck Reflex, Asymmetrical Tonic Neck, Hands Grasp, Hands Pulling, Babkin Palmomental.
Improvement in normalization of the record results may lead to letting go the rigid sensory-motor patterns, depending on muscle tone dysregulation, and also improvement in plasticity and flexibility of the body resulting in new motor-cognitive and behavioral patterns and support in building of the motor control, self-awareness and increased communication abilities including expression of own needs and cognitive motivation in interactions. Children with CP with changes in Dorsolateral Prefrontal Cortex after the MNRI Program were also showing improved motor memory, ability to focus for longer time and showing deeper comprehension, long-term memory, easier processing, better stress management, less sensory-motor reactivity. They had certain improvement in motivation, attention, and interest in more complicated movements.
Electrodes activity recording from postcentral gyrus: C3/C4- Areas 1, 2, 3
Findings: Analysis of the results of brain mapping after the MNRI program further showed the notable improvements in the electrical potential in the Postcentral Gyrus of the brain in area C3/C4 (Areas 1, 2, 3) presenting the somatosensory cortex responsible for perception and processing of sensory input following from exteroceptors of the skin and muscular-tendinous system [Figure 11].
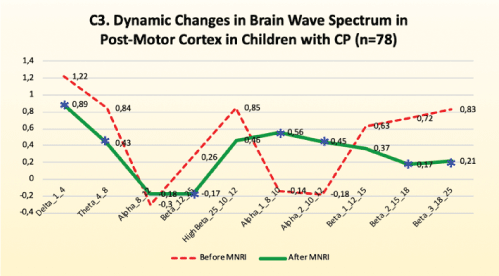
Figure 11: C3. Dynamic changes in brain wave spectrum in post-motor cortex in children with CP (n=78).
Improvements are noted in the following:
• Significant decrease of the electrical potential recording in the range of 12-15 Hz (sensorimotor rhythm), which suggests improvement in normalization of the sensory-motor integration and decrease in motor reflexive re-activity as the proper reduction in fast frequencies for the brain waves took place.
• Decrease of hyperactivity in these somatosensory areas seen in bettering the postural control, motor coordination, and also in sensory-motor-cognitive multitasking.
• Better tactile reception and perception-better response for activation of specific reflex receptive areas/fields, better interpretation of different textures and shapes, ability for discrimination of qualities of objects, sensory binding of tactile perception with audio-visual integration), selective perception of parts of own body (BA 13 insula, perception+metric control C3-T7).
• Coordination between tactile, proprioceptive, and vestibular systems.
Reflex patterns functions, particularly for Galant, Tonic Labyrinthine, Hands Grasp, Babkin Palmomental, Babinski, Foot DorsiFlexion.
Electrodes activity recording from angular gyrus (including part of Wernicke’s area): P4 Brodmann Area 39 [Figure 12]

Figure 12: P4. Dynamic changes in brain wave spectrum in Wernicke area in children with CP (n=78).
Findings: Analysis of the results of the brain mapping in the Wernicke area (P4) prior and after the MNRI intervention program in the Study Group shows certain improvements in the electrical potential, results, particularly in the following:
Improvements are noted in the following:
A significant decrease in median for: delta, theta, beta, high-beta, beta1, beta2, beta3, which means the normalization of the record, which was expressed in more interests in interaction, increased ability to expressive language, attention and memory, number processing and spatial-cognitive retrieval.
Some increase for the alpha wave spectrum in the range of 8-10 Hz took place, which was seen in increased calmness, relaxation, inner peace, also presence and self-awareness and ability for longer-term focusing on gross and fine motor tasks.
The normalization of the EEG record resulted in an increase of the ability for language development: to recognize and to connect sounds into syllables, syllables into words and words into phrases, to use more comprehensive sentences in their speech.
Less of reactive response (startle) for unexpected or too intense audio and audio-visual stimuli.
Reflex patterns functions, particularly for Asymmetrical Tonic Neck, Symmetrical Tonic Neck, Stapedial/Auditory, Babkin Palmomental, and fear Paralysis.
Electrodes activity recording from primary auditory association cortex: T3/T4-Areas 41 and 42
Findings: Normalization of the EEG record in the T3/T4 area, the which presents Primary Auditory and Associative Cortex, was seen in children’ with CP results after the MNRI intervention [Figure 13].
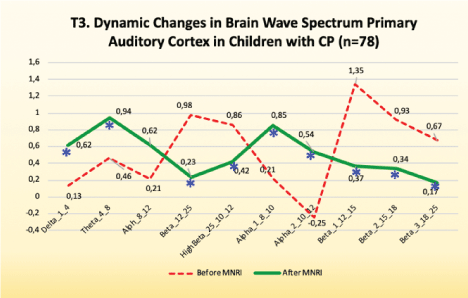
Figure 13: T3. Dynamic changes in brain wave spectrum primary auditory cortex in children with CP (n=78).
Improvements are noted, particularly, in the following:
High amplitudes of fast wave frequencies in this area showed the tendency for normalization, also seen in fewer epilepsy episodes in children that had them.
Calming down, being more present, better ability to relax, decrease in muscle hypertone and improvement in response to more intense (loud, unexpected) sounds, less anxiety.
Increase in tolerance for audial reception and perception.
Better ability in sound orientation in space, in sensory adjustment of auditory and visual perception.
Biomechanics of such reflex patterns as: Stapedial, Auditory Fear Paralysis, Moro, Auditory Orientation, also Binaural hearing.
Reflex patterns functions, particularly for Asymmetrical Tonic Neck, Symmetrical Tonic Neck, Stapedial/Auditory, Babkin Palmomental, fear Paralysis.
Cz-Area 5-Somatosensory processing and association cortex
Findings: statistically significant changes in this area of secondary somatosensory and association cortex responsible for functions of: decoding of the input from the exteroceptive system, processing and forming the perception, linking processes of tactile sensory reception with other sensory modalities-proprioceptive, vestibular and visual and auditory [Figure 14].
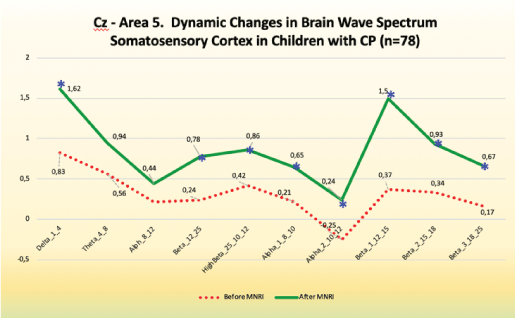
Figure 14: CZ- Area 5. Dynamic changes in brain wave spectrum somatosensory cortex in children with CP (n=78).
Improvements are noted, particularly, in the following:
Decrease in slow frequencies for Delta and Theta.
Increase in fast frequencies of Beta, High Beta, Beta 1 and Beta 2, and Beta 3.
Tendency for increase in slow frequencies for Alpha 1 and Alpha 2. The increase in low frequency waves in the range of -0.60 to 0.4 of absolute power range and increase at the same time in the high frequency waves in the range of 0.00 to 1.00 as seen in better regulation of sensory reception and perception, balance in inhibition-arousal (calming down, inner peace), control of behavior and cognitive processes, focusing skills, awareness of motor tasks, comprehension, comparison, analysis, language.
Reception/perception of sensory stimuli with less of hypo-or hyper sensitive response, ability of associating kinesthetic and motor-visual activity with movements.
Increase of coordination of integrative functions and better control of the sensory-motor and cognitive multitasking.
Better control and coordination of limb movement, stretch and contraction of muscles-tendons in extending, rotational movements and flexion, better tactile learning and motor memory seen in following instruction given in exercises for the whole body and its segments.
Differentiation of texture and size of hand-held objects.
• Pain sensation, less of self-damaging while moving and manipulating the objects.
The maps also show several other positive changes:
• A major improvement in frontal delta and alpha power overexpression
• Partial improvement in the beta and high beta power
• An increase in both frontal alpha and frontal and central beta/ high beta power compared to initial increase in frontal delta power.
• Certain changes in abnormalities in coherence and phase lag.
Individual case example before and after the MNRI processes
The below presented summary maps show an example of positive changes in the brain wave spectrum in an individual case before and after the MNRI therapy modality [Figures 15 and 16].
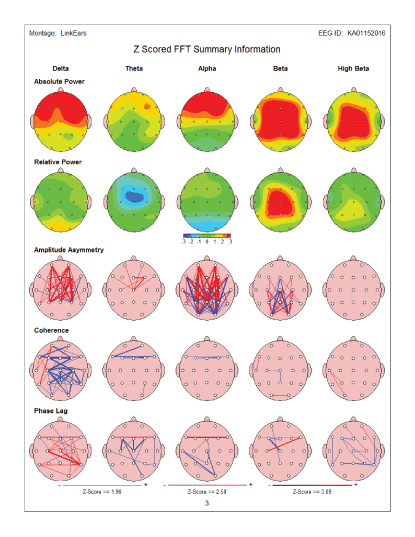
Figure 15: Summary map: Pre-test - before MNRI Therapy: Showed increase in frontal delta power as well as Increase in both frontal alpha and frontal and central beta/high beta power. There are also abnormalities present incoherence and phase lag.
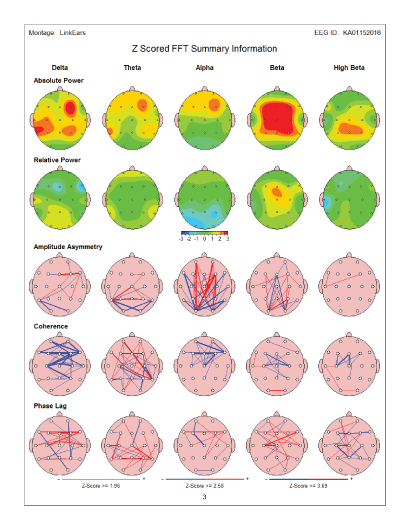
Figure 16: Summary map: Post-test - after MNRI Therapy: QEEG maps after therapy showed a major improvement in frontal delta and alpha power over- expression. In addition, beta and high beta power partial improvement was noted.
Global measures for CNS processes
The QEEG maps results were also analyzed for global measures for CNS processes: 1) under arousal, 2) inhibition, 3) over arousal, 4) executive processing, 5) memory processing, 6) verbal processing, 7) visual processing, 8) reading comprehension, 9) digital/math comprehension, and their symptoms (See example below).
The summarized analysis of QEEG Brain Mapping by symptoms criteria allowed to register the positive changes in 93.75% (45 out of 48) of symptoms, and particularly: A) 52.1% (25) of them changed from severe to moderate, B) 33.33% (16) from severe to much higher functioning and C) 8.33 % (4) from moderate to much higher functioning, and D) 6.25% (3)-no changes.
These results demonstrate extremely high effect of the symptoms changes considering the fact that they were reached within 8 days of intensive treatment program [Table 5] [Figure 17].
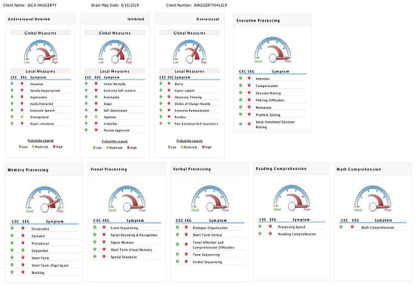
Figure 17: These results demonstrate extremely high effect of the symptoms changes considering the fact that they were reached within 8 days of intensive treatment program.
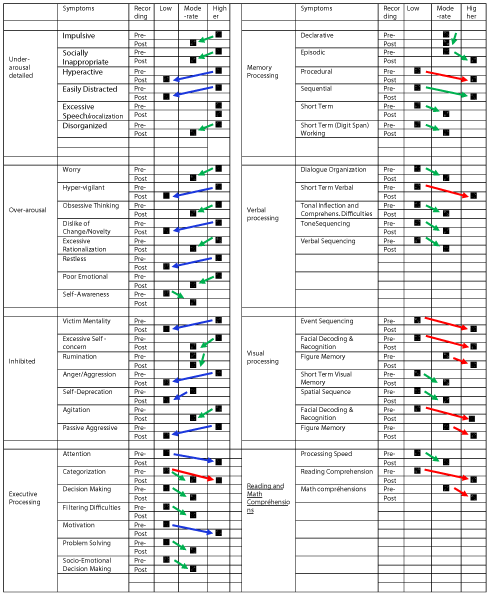
Table 5: Summarized results of QEEG brain mapping changes by symptoms criteria.
The analysis of the Theta-Beta Ratios. The analysis of the theta/beta ratio biomarker reflecting the pre frontally mediated attention and conscious control demonstrated improvement in the brain maturity and cognitive processes of children with CP in Study Group (n=78). Particularly the ratio changed from average 5.24 before the MNRI intervention to average 3.86 after (p>0.05) [Figures 18 and 19].
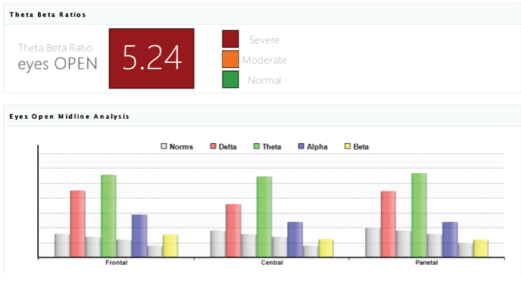
Figure 18: The Theta-Beta Ratios biomarker result in the brain maturity and cognitive processes of children with in children with CP in Study Group (n=78) of average 5.24.
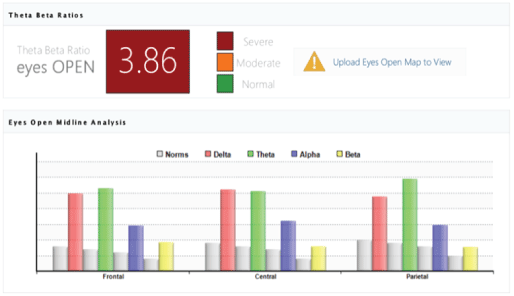
Figure 19: The Theta-Beta Ratios biomarker analysis results: Improvement in the brain maturity and cognitive processes of children with CP in study group (n=78) from average 5.24 before the MNRI intervention to average 3.86 after (p>0.05).
Analysis of changes in the brain wave spectrum on level of Absolute Power (1), Relative Power (2), Coherence (3), Interhemispheric Asymmetry (4), and Phase lag (5)
The comparative pre-and post-evaluation of the QEEG results analysis also demonstrated a very good effect of the MNRI Neuromodulation Program on different aspects of the brain wave spectrum. Particularly, these changes were seen in the following dimensions:
Absolute Power - the amount of activity from slowest (left)-Delta to fastest (right)-Beta frequency of brain waves was noted in 73.08 %/57 of children after the MNRI Program, which can be interpreted as the tendency for better focusing and awareness, presence and regulation and maturity of the brain wave spectrum organization. Activity in each frequency band compared to a normative database moved to more positive changes from prior noted deviations in the brain wave spectrum. The results for each frequency band are represented in green color as an average activity, and red-a significant increase in activity, yellow-moderate increase in activity compared to the norm, and blue as a significant decrease [Figure 20].
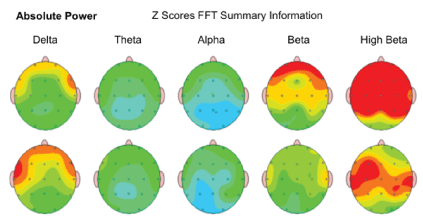
Figure 20: Absolute Power: Amount of activity within a specific frequency band of brain waves in the study subject in order of slowest (left)-Delta to fastest (right) Beta.
Relative Power-the improvement in relative level of activity within a specific frequency band compared to all the other frequency bands was noted in 67.95 %/53 of children after the MNRI Program. Level of relative activity in each frequency band compared to the norm shows positive dynamic in shifting from the abnormalities. The results for each frequency band are presented by the topographic activity maps (see the example); green is the color representing average activity, while red-a large increase in activity, yellow-moderate increase of activity, and blue-a large decrease [Figure 21].
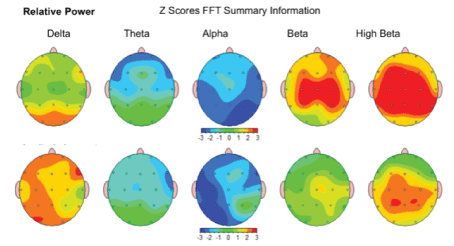
Figure 21: Relative Power: Amount of activity within a specific frequency band of brain waves in the study subject compared to all the other frequency bands; in order of slowest (left)-Delta to fastest (right) Beta.
Coherence is the marker of similarity in EEG waves over different brain areas, i.e., the timing of activity in one area vs. another show certain improvement in 63.88%/42 of children’s brain maps after the MNRI Program. Coherence in each frequency band compared to the norm showed noted positive shift form abnormalities (see the topographic connection maps). This is presented particularly by thick lines representing bigger abnormalities compared to normred referring to increased coherence, and blue linked to decreased coherence [Figure 22].
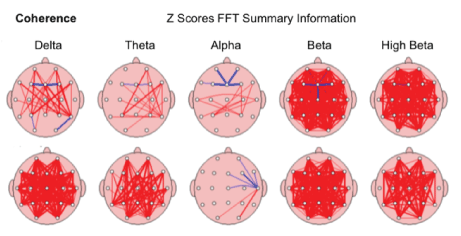
Figure 22: Coherence: The similarity in EEG waves over different areas of the brain (i.e., the timing of activity in one area compared to another) in the study subject; in order of slowest (left)-Delta to fastest (right) Beta.
Interhemispheric Asymmetry-showed improvement in relationship between the amounts of activity in one area of the brain compared to another took place in brain maps of 65.38%/51 of children after the MNRI Program. The analysis has demonstrated the fact of decrease of abnormalities in Asymmetry markers in each frequency band compared to the norm database. The topographic connection maps on fig. below show the thick lines representing larger deviations from “normal” red refers to increased asymmetry, while blue refers to decreased asymmetry [Figure 23].
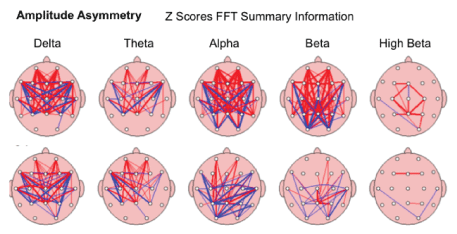
Figure 23: Amplitude Asymmetry: The relationship between the amounts of activity in one area of the brain compared to another in the study subject; in order of slowest (left)-Delta to fastest (right) Beta.
Phase lag is the measure of level of functional connectivity. The Phase Lag Index (PLI) is a measure of asymmetry of the distribution of phase differences between two signals [Figure 24].
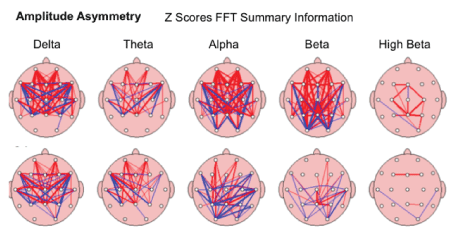
Figure 24: Phase lag: The estimation of the synaptic integration times (the speed of shared information) between two sites of the brain in the study subject; in order of slowest (left)-Delta to fastest (right) Beta.
The MNRI Program demonstrated the regulative effect on brain maps of children with CP, whose diagnosis of brain damage traditionally is considered a limiting factor for changes. The MNRI corrective work opens new perspectives in the work with children with CP showing positive effect of reflex neuromodulating techniques on different dimensions of the brain wave spectrum normalization, particularly on Absolute Power, Relative Power, Coherence, Interhemispheric Asymmetry, and Phase lag, which support brain wave spectrum normalization, the increase of sensory-motor integration, stress resilience and neuroplasticity.
QEEG evaluation of children with CP and variety of other neurological conditions has been shown to be a very useful tool for screening the issues on brain wave spectrum level to determine next the possible degrees of changes after intervention programs [95].
The study results of current brain maps allow us to state that the therapy modality using MNRI in individuals with CP leads to reorganization, improvement of regulation of the brain waves in all its markers, and leads to partial, however to overall normalization of spontaneous brain electrical activity.
This was regularly observed in changes of over expressed activity of delta and theta frequency toward improvement in alpha, beta and high beta over expressed activity. This normalization of QEEG activity is correlated with changes in the neurotransmitters of CP children and the reflex neuromodulation and integration effect of the MNRI intervention.
This result has been supported by data showing consistent positive clinical responses noted after MNRI therapy. Our observations are coherent with the findings of other scientists that noted changes in EEG brain mapping results in children with CP during studies of the motor system [96].
Clinical observation by helping professionals (PTs, doctors, OTs, SLPs, special educators, psychologists and others) as well as parents and caregivers shows that, after the MNRI program, children with CP and other types of neurological dysfunction improved their motor programming and planning, balance, and postural control [34]. This neuromodulation program allows them to improve their motor coordination, muscle strength, precision of gross and fine movements, space-time orientation, speed of perception and response, better presence of mind, easier and prolonged focusing, better memorizing, and improvement in receptive and expressive language.
Analysis of the MNRI effects on reflex sensory-motor integration in children with CP showed that their reflex patterns improved significantly. This result is an indication that the neurosensory development and overall functioning of these children can be improved in spite of their neurological pathologies and brain damage. The improvements were also noted in: sensory-motor integration, behavior and emotional regulation, communication; stress resilience; physical health; school skills and achievement motivation increase. The study of effectiveness of the MNRI program for children with CP serves as a potential example and best practices for children with other neurological deficits and learning disabilities.
The results of this study also indicate that changes in impaired reflex patterns that follow after the MNRI therapy targeting a reflex components “training” do not happen spontaneously in children with CP, and that knowledge of the nature of “normal” reflex and dysfunction/pathology is essential. Furthermore, specialized work targeting the sensory-motor coordination and integration of reflex patterns needed, vs. the traditional concept of inhibiting “retained” reflexes. Reflex as units and a function of the nervous system [4,46] must be trained within its “normal model” instead of “fighting” with a “dysfunction/pathology”, by other words the change of the concept in treatment practice of a reflex pattern must be done from symptomoriented to a reason-solving approached. The long-term clinical observations and the collection of scientific data, done by the authors of this article allows to state that the MNRI program aimed at serve for a basic physiological reflex circuits must be a preliminary therapy, prior to other types of therapy modalities such as physiotherapy, occupational therapy, speech pathology therapy, or sensory integration to help pave the nerve pathway for success brain development [49].
Future MNRI research will be oriented at technical information concerning the neurophysiological mechanism and biomechanical aspects of a reflex patterning process that the MNRI method can potentially activate though corrective tools using the reflex ‘model scheme’ to improve the reflex circuit parameters and sensory-motor pattern functions in children with CP and other neurological deficits. There are particular methods and techniques to target automatic functions to support their maturation and strengthen the level of the subcortical structures of the brain and lower motor neuron functions. This, in turn, will allow to support the higher executive functions such as conscious motor coordination and postural control, regulation of behavior and emotions, cognitive processes (comparison, analysis, comprehension, language), and personality development, as this has been proven in work with78 individuals with brain damage diagnosed as CP.
Neurotransmitters data analysis on changes in children with cp after the MNRI reflex neuromodulation program
The test panel of twelve neurotransmitters in the Study Group of children with CP (n=78) was compared prior and after the intensive MNRI Reflex Integration intervention and also compares with the Control Group of children with neurotypical development (n=117).
The urine collection per manufacturer’s specifications was done on the first day of the intervention and again at the last day of the conference. The urinalysis test utilized was the #9123 extended panel, testing twelve neurotransmitters, particularly: epinephrine, norepinephrine, 3, 4-dihydroxyphenylacetic acid (DOPAC), dopamine, serotonin, 5-HIAA, GABA, PEA, glycine, histamine, taurine, and glutamate.
The participants prior to the sample collection were to fast for 8 hours and abstain from tobacco, avoid strenuous workouts, and not to take supplements or medications (unless instructed otherwise by a physician). The urine was the second urine of the morning following two hours after wake up while still fasting. The needed volume was two 5 ml tubes to accommodate the instruction for lab analysis. One vial contained a stabilizer as specified to maintain the integrity of the sample. The samples were kept refrigerated until processed in the reference lab. All the urine samples were processed by the Neuroscience Lab (WI), which had no vested interest in MNRI. Independent scientists analyzed the results and compiled statistics. They were not given any details regarding the treatment or suspected outcomes to exclude the bias approach in interpreting the results [Table 6].
Table 6: Neurotransmitters changes in children with cerebral palsy in the Study Group (n=78; age: 2- 19 y.o.).
The Neurotransmitters analysis in the Study Group of cerebral palsy children showed: Epinephrine had -0.1 effects. Norepinephrine had0.2 effects. 5-HIAA had 0.2 effects. DOPAC had 0.3. Dopamine had 0.4. GABA had 0.1. Glutamate had 0.2. Glycine had-0.3. Histamine had 0.5. PEA had 0.3. Serotonin had 0.5, and Taurine had 0.1. All results are in the category of small effects, except for histamine and serotonin which also fell into the lower tier of medium effect size. Data shows certain improvements in results in the study group of children with CP, and particularly, the impact of the MNRI program in changes in glycine and norepinephrine, at a 0.2-0.3 effect which can be categorized as a small effect according to Cohen’s method. Glycine plays a role with the inflammatory response and immune response within the whole organism. Nevertheless, the impact of DOPAC, Dopamine, Epinephrine, GABA, Glutamate, and Taurine was noted in ability of children with CP to: focus (especially in visual activities); ability for longer time of cognitive involvement for hands-on tasks; better balanced excitation and inhibition seen in the movement control (easier, faster, more flexible); better mood control and less of startle response, better stress management that can be interpreted by decrease of taurine level; easier interaction and articulation and speech.
These study findings suggest that MNRI Therapy Program positively impacts neurological and endocrine system homeostasis through neurotransmitters and stress hormone regulation. The results indicated the reduction of high excitability and several excitatory neurotransmitters within the nervous system in children with CP disorder, also activation of neurotransmitters affecting the decrease of inflammation warrants further research for potential clinical significance.
The MNRI Program demonstrates the regulative effect on brain maps, neurotransmitters regulation and reflex maturity of children with CP through the use of the concept and restoration techniques for ‘stressed’ and dysfunctional reflex circuits.
All three of evaluation methods showed substantial positive changes in children with CP in corresponding areas after the MNRI treatment, particularly:
a) Reflex post-Assessment demonstrated that improvement in 66.7%/26 reflex patterns (out of 30 patterns) moving them from pathology and severe dysfunctional level to moderate dysfunctional or even low level of development (p<0.05) within 8 days of the intensive MNRI Program, indicating the changes in the sensorymotor development of CP children, whose diagnosis of brain damage traditionally is considered a limiting factor for changes. The MNRI corrective work opens new perspectives in work with children with CP showing positive effects of reflex neuromodulation techniques. The MNRI corrective work allows paving and rerouting nerve pathways to bring them into a state of proper neurophysiological functioning in order to improve the balance of the excitation-inhibition processes of the nervous system, also increase stress resilience and neuroplasticity
b) The QEEG study of children with CP identified 83, 33% (n=65 out of 78) of them as having QEEG abnormalities. All post-brain map results after the MNRI interventions showed improvement in 79.5% (n=62 out of 78) study participants. The level of an improvement after the MNRI intervention varied from subject to subject and only a small number of participants-8.97% (n=7) showed no evidence of significant brain mapping improvement. Children with improved results displayed a reorganization of spontaneous brain electrical activity observed in the marked increase of global delta, beta, and high beta powers as well as an increase in frontal/temporal theta and alpha powers took place meaning that the abnormalities in hyper/hypocoherence and phase lag improved similar to those of the previous study done in the MNRI [23,32]. Analysis of changes in dimensions of the brain wave spectrum-Absolute Power, Relative Power, Coherence, Interhemispheric Asymmetry, and Phaselag-shows its tendency to normalization also improve sensory-motor integration, increase stress resilience and neuroplasticity
c) The pre and post-analysis of 12 neurotransmitter markers after MNRI Program showed improvement in: a) regulating effect of excitatory and inhibitory neurotransmitters, b) enhancement of stress regulation (taurine decrease); c) an anti-inflammatory effect (improving cytokinesis), d) restoration tendency for the nerve fibers (serotonin as a modulator for glutamate), and d) hormonal changes supporting the muscle tone regulation and motor control support. This Study Group noted a small increase in several excitatory neurotransmitters. This finding warrants further research for potential clinical significance. These findings demonstrate that the MNRI Program positively affects the neurological and endocrine system homeostasis through neurotransmitter and stress hormone modulation. These synergistic effects influence optimization of immune function as well thus influencing the physiology on multiple levels.
Long-term clinic experience working with the MNRI tools shows the need of 4-6 years of work in the Program intervention to assure the development of reflex patterns affecting development of main sensorymotor milestones and neurodevelopment capable to enhance the regulative functions of postural control, motor coordination, sensorymotor integration to significantly higher functional level. This may be connected with therapeutic effect of the MNRI method assuring the progress for cases of neuromotor disorders originated in the central nervous system, which similarly is noted by other authors concerning the impact of the motor training on brain wave spectrum of children with CP [99].
We acknowledge scientists for carrying out this multi-focused study. It was approved by the New England IRB (USA). Its principal investigators and scientists-S. Masgutova, Ph.D., Dr. J. Koberda, MDS, Prof., P. Shackleford, Ph.D. (USA), P. Sobaniec, Ph.D., Katarzyna Nowak (Poland), Dr. N. Akhmatova, Prof. (Russia) and et al.-have studied the group of 310 children (2-19 y.o.) with different neuro deficits including children with the CP (n=78) during the MNRI trainings programs at. Family Conferences
Special acknowledgement for SMEI Publication Specialist-Sally Averkamp.
All specialists leading the evaluations were certified by the NIH (National Institute of Health of, Office Extra mutual Research) “Protecting Human Research Participants” in 2012-2015. All participants were assigned codes to protect anonymity. Receipts of informed consent were received from all participants’ parents or legal guardians. MNRI Assessments were conducted and treatment administered by designated Specialists or MNRI Core Specialists who have successfully completed the requirements for Continuing Professional Education in MNRI and clinical hours (www. MasgutovaMethod.com).
- Westermann J, Hubl W, Kaiser N, Salewski L (2002) Simple, Rapid and Sensitive Determination of Epinephrine and Norepinephrine in Urine and Plasma by Non-Competitive Enzyme Immunoassay, Compared With HPLC Method. Clin Lab 48: 61-71. [Ref.]
- Panholzer TJ, Beyer J, Lichtwald K (1999) Coupled-column Liquid Chromatographic Analysis of Catecholamines, Serotonin, and Metabolites in Human Urine. Clin Chem 45: 262-268. [Ref.]
- Kågedal B, Goldstein DS (1988) Catecholamines and Their Metabolites. J Chromatogr 429: 177-233. [Ref.]
- Sechenov I (1965) Reflexes of the brain. In: Gibbons G (eds) MIT Press, United States. [Ref.]
- Shackleford G, Mahdi M, Ren X, Moats R, Erdreich-Epstein A (2015) PTPS-05 Pid1 Negatively Regulates Growth of Intracranial Medulloblastomas In Vivo in Bartel Mice. Neuro-Oncology 17: 180. [Ref.]
- Palisano RJ, Hanna SE, Rosenbaum PL, Russell DJ, Walter SD, et al. (2000) Validation of a Model of Gross Motor Function for Children With Cerebral Palsy. Phys Ther 80: 974-985. [Ref.]
- Deiss T, Meyers R, Whitney J, Bell C, Tatarinova Т, et al. (2019) Physiological Markers and Reflex Pattern Progression in Individuals with Neurodevelopmental Deficits Utilizing the MNRI Method. Neurosci Med 10: 30-54. [Ref.]
- Wright J (2017) Socialism and the Experience of Time: Idealism and the Present in Modern France. Oxford University Press: 352. [Ref.]
- Palisano RJ, Russell D, Rosenbaum P, Walter S, Gemus M, et al. (1997) Development Of Gross Motor Function In Children With Down Syndrome. Pediatric Physical Therapy 9: 197. [Ref.]
- Wood E, Rosenbaum P (2000) The Gross Motor Function Classification System for Cerebral Palsy: A Study of Reliability And Stability Over Time. Dev Med Child Neurol 42: 292-296.
- Zablocki BD (1998) Exit Cost Analysis: A New Approach to the Scientific Study of Brainwashing. Nova Religio: J Alternative Emergent Religions 1: 216-249. [Ref.]
- Arneson CL, Durkin MS, Benedict RE, Kirby RS, Yeargin-Allsopp M, et al. (2009) Prevalence of Cerebral Palsy: Autism and Developmental Disabilities Monitoring Network, Three Sites, United States, 2004. Disabil Health J 2: 45-48. [Ref.]
- Lord C (2008) Faculty Opinions recommendation of Prevalence of autism spectrum disorders-Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 1057-1062. [Ref.]
- Pakula AT, Van Naarden Braun K, Yeargin-Allsopp M (2009) Cerebral Palsy: Classification and Epidemiology. Phys Med Rehabil Clin N Am 20: 425-452. [Ref.]
- Paneth N, Hong T, Korzeniewski S (2006) The Descriptive Epidemiology of Cerebral Palsy. Clin Perinatol 33: 251-267. [Ref.]
- Winter S, Autry A, Boyle C, Yeargin-Allsopp M (2002) Trends in the Prevalence of Cerebral Palsy in a Population-Based Study. Pediatrics 110: 1220-1225. [Ref.]
- Shin LM, Rauch SL, Pitman RK (2001) Symptom provocation studies: The example of anxiety disorders. In: Dougherty DD, Rauch SL (eds) Psychiatric neuroimaging research: Contemporary strategies. Arlington, American Psychiatric Publishing, USA 101-124. [Ref.]
- Data and Statistics for Cerebral Palsy. [Ref.]
- Masgutova S (2016) Post-Trauma Recovery in Children of Newtown, CT using MNRI Reflex Integration. J Trauma Stress Disor Treat 5: 5. [Ref.]
- Masgutova S, Akhmatova N (2004) The integration of dynamic and postural reflexes of the entire mobility system. MINK, Warsaw, Poland.
- Masgutova S, Regner A (2009) The development of child’s speech in the light of sensory integration. Continuo, Wrocław, Poland.
- Pilecki W, Masgutova S, Kowalewska J, Masgutov D, Akhmatova N, et al. (2012) The impact of rehabilitation carried out using the Masgutova Neurosensorimotor Reflex Integration method in children with cerebral palsy on the results of brain stem auditory potential examinations. Adv Clin Exp Med 21: 363-371. [Ref.]
- Pilecki W, Kipiński L, Szawrowicz-Pełka T, Kałka D, Masgutova S (2013) Spectral brain mapping in children with cerebral palsy treated by the Masgutova Neurosensorimotor Reflex Integration method. J Neurol Sci 333: e550. [Ref.]
- Michalowicz R (2001) Childhood cerebral palsy. WydawnictwoLekarskie PZWL, Warszawa, Pl.
- Masgutova S, Akhmatova N (2004) Reflexes re-patterning exercises. Integration of dynamic and postural reflexes into the whole body movement system.
- Reimers S (2020) De M-T. Fundamentals.
- Masgutova SK, Akhmatova NK, Lebedinskaya OV (2013) Clinicalimmunological assessment of therapy effect of the neuro-sensorymotor integration program of reflex patterns in airway chronic inflammatory diseases. Front Immunol 4. [Ref.]
- Renard-Fontaine I (2017) Effect of Reflex Neuromodulation on an Infant with Severe Amniotic Band Syndrome: A Case Report on the use of MNRI Techniques for Physical Therapy. Int J Neurorehabilitation 4: 248. [Ref.]
- Murphy TH, Corbett D (2009) Plasticity during Stroke Recovery: From Synapse to Behaviour. Nat Rev Neurosci 10: 861-872. [Ref.]
- Akhmatova NK, Masgutova SK, Shubina IZ, Akhmatov EA, Khomenkov VV, et al. (2015) Immunological Effects of Masgutova Neurosensorimotor Reflex Integration in Children with Recurrent Obstructive Bronchitis. Int J Neurorehabilitation 2: 166. [Ref.]
- Wisniewski KE (1990) Down Syndrome Children Often Have Brain With Maturation Delay, Retardation of Growth, and Cortical Dysgenesis. Am J Med Genet Suppl 7: 274-281. [Ref.]
- Koberda JL, Akhmatova N, Akhmatova E, Bienkiewicz A, Nowak K, et al. (2016) Masgutova Neurosensorimotor Reflex Integration (MNRI) Neuromodulation Technique induces Positive Brain Maps (QEEG) Changes. J Neurol Neurobiol 2. [Ref.]
- Masgutova S, Sadowska L, Kowalewska J, Masgutov D, Akhmatova N, et al. (2016) Use of a Neurosensorimotor Reflex Integration Program to Improve Reflex Patterns of Children with Down Syndrome. J Neurol Neurosci 6: 4. [Ref.]
- Rentschler M AS (2015) Reflexes: Portal to Neurodevelopment and Learning-A Collective Work. Svetlana Masgutova Educational Institute, Orlando.
- Krefft A (2007) Diagnosis of functions of invisible phenomena (Statistic Mathematic Analysis). Oficyna Wydawnicza Politechniki Wrocławskiej.
- Akhmatova NK, Masgutova S, Lebedinskaya OV, Akhmatov EA, Shubina I (2015) Immunological efficiency of MNRI program a t treatment of respiratory diseases. Front Immunol. [Ref.]
- Selye H Md (1974) stress without distress. New York: The New Ameri-can Library.
- Anokhin PK (1973) Fundamental questions of the general theory of functional systems. Principles of system organization of functions, Nauka, Moscow: 5-61.
- Musajanova R (2011) Pathogenetic approaches to the treatment of children with chronic pneumonia. Med Princ Pract 7: 87-89.
- Bremner JD (2006) Stress and Brain Atrophy. CNS Neurol Disord Drug Targets 5: 503-512. [Ref.]
- Vermetten E, Bremner JD (2002) Circuits and systems in stress. I. Preclinical studies. Depress Anxiety 15: 126-147. [Ref.]
- Pitman RK, Shin LM, Rauch SL (2001) Investigating the Pathogenesis of Posttraumatic Stress Disorder With Neuroimaging. J Clin Psychiatry 62 Suppl 17: 47-54. [Ref.]
- Zablocki KJ (1998) Childhood cerebral palsy in theory and therapy. WydawnictwoAkademickie “Żak” Warszawa, Pl.
- Bobath K, Bobath B (1984) Management of the motor disorders of children with cerebral palsy. London: Mac Keith Pr.
- Souchay C, Guillery-Girard B, Pauly-Takacs K, Wojcik DZ, Eustache F (2013) Subjective Experience of Episodic Memory and Metacognition: A Neurodevelopmental Approach. Front Behav Neurosci 7: 212. [Ref.]
- Pavlov IP (2010) Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. Ann Neurosci 17: 136- 141. [Ref.]
- Haines DE (2013) Fundamental Neuroscience for Basic and Clinical Applications, with STUDENT CONSULT Online Access. 4th Edition. [Ref.]
- Asratian EA (1983) Selected works. Reflexive theory of high nerve system activity. Science, Moscow.
- Bernstein N (1997) Biomechanics and Physiology of Movement. Moscow, RU: Moscow-Voroniez.
- Akhmatova N, Akhmatova E (2017) Influence of MNRI on the Immune Status of Children with Down Syndrome. J Clin Cell Immunol 8: 483. [Ref.]
- Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, et al. (2005) Proposed Definition and Classification of Cerebral Palsy, April 2005. Dev Med Child Neurol 47: 571-576. [Ref.]
- Wimalasundera N, Stevenson VL (2016) Cerebral palsy. Pract Neurol 16: 184-194. [Ref.]
- O’Shea TM (2008) Diagnosis, Treatment, and Prevention of Cerebral Palsy. Clin Obstet Gynecol 51: 816-828. [Ref.]
- Spittle AJ, Orton J (2014) Cerebral Palsy and Developmental Coordination Disorder in Children Born Preterm. Semin Fetal Neonatal Med 19: 84-89. [Ref.]
- Yarnell J, O’Reilly D (2012) Epidemiology and Disease Prevention: A Global Approach. OUP Oxford. [Ref.]
- Chen XY, Wolpaw JR (2002) Probable corticospinal tract control of spinal cord plasticity in the rat. J Neurophysiol 87: 645-652. [Ref.]
- Clowry GJ (2007) The dependence of spinal cord development on corticospinal input and its significance in understanding and treating spastic cerebral palsy. Neurosci Biobehav Rev 31: 1114-1124. [Ref.]
- Eyre JA, Taylor JP, Villagra F, Smith M, Miller S (2001) Evidence of Activity-Dependent Withdrawal of Corticospinal Projections During Human Development. Neurology 57: 1543-1554. [Ref.]
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, et al. (2008) Development and Reliability of a System to Classify Gross Motor Function in Children With Cerebral Palsy. Dev Med Child Neurol 39: 214-223. [Ref.]
- Sadowska L (2001) Vaclav Vojta’s Neurokinesiological Concept for the Diagnosis and Therapy of Children With Disturbances of Motor Development. Ortop Traumatol Rehabil 3: 519-526. [Ref.]
- Sadowska L DM (2012) Neurophysiological basis for diagnosis and treatment of children with developmental disorders. Ed. WSzM Warsaw, PL.
- Sadowska LL, Masgutova S, Kowalewska J, Filipowski H (2008) Reflex Integration of Children with Down syndrome. Education 17: 18-10.
- Masgutova SK (2006) Neuro-Kinesiology as the support for development and learning. Children with Learning Challenges and ADHD. Materials of Polish National Conference: “Modern Methods of Stimulation of Movement Development and Learning in Children with Difficulties in Learning, ADHD and Autism. 236-253.
- Masgutova S MD (2005) The Integration of Facial Reflexes Using Svetlana Masgutova Method. Techniques Supporting the Development of Motor Skills and Speech. Warsaw, PL: MINK.
- Roohi-Azizi M, Azimi L, Heysieattalab S, Aamidfar M (2017) Changes of the Brain’s Bioelectrical Activity in Cognition, Consciousness, and Some Mental Disorders. Med J Islam Repub Iran 31: 53. [Ref.]
- Mental Health Daily. [Ref.]
- Neurofeedback, Biofeedback & Brain therapy London-Brainworks Neurofeedback: the UK’s trusted leader since 2007. [Ref.]
- Vojta V (1964) Current Methods of Rehabilitation in the Treatment of Motor Disorders in Perinatal Encephalopathy. Cesk Neurol 27: 81-86. [Ref.]
- Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000) Molecular cell biology. 4th Edition, National Center for Biotechnology Information, Bookshelf. [Ref.]
- What are neurotransmitters? [Ref.]
- Selye H (1987) Stress without distress (pathway). New York.
- Virella G (1998) Introduction to Medical Immunology 4th (eds). Marcel Dekker, the University of Michigan: 651. [Ref.]
- Ziemssen T, Kern S (2007) Psychoneuroimmunology--cross-talk Between the Immune and Nervous Systems. J Neurol 254: II8-II11. [Ref.]
- Spitzer NC (2010) How GABA generates depolarization. J Physiol 588: 757-758. [Ref.]
- Park HJ, Kim CH, Park ES, Park B, Oh SR (2013) Increased GABA-A Receptor Binding and Reduced Connectivity at the Motor Cortex in Children With Hemiplegic Cerebral Palsy: A Multimodal Investigation Using 18F-fluoroflumazenil PET, Immunohistochemistry, and MR Imaging. J Nucl Med 54: 1263-1269. [Ref.]
- Lee JD, Park HJ, Park ES, Kim DG, Rha DW, et al. (2007) Assessment of Regional GABA(A) Receptor Binding Using 18F-fluoroflumazenil Positron Emission Tomography in Spastic Type Cerebral Palsy. Neuroimage 34: 19-25. [Ref.]
- Yuan HB, Cheng LY, Yin F, Zhang GX, Peng J, et al. (2008) Levels of Amino Acids in Cerebral Spinal Fluid in Children With Cerebral Palsy. Zhongguo Dang Dai Er Ke Za Zhi 10: 475-477. [Ref.]
- Pregnolato S, Chakkarapani E, Isles AR, Luyt K (2019) Glutamate Transport and Preterm Brain Injury. Front Physiol 10: 417. [Ref.]
- Samuels ER, Szabadi E (2008) Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part I: Principles of Functional Organisation. Curr Neuropharmacol 6: 235-253. [Ref.]
- Shackleford P, Roy V, Cameron A, Ortego L, Marks T, et al. (2017) Flood Trauma Survival and Recovery Using MNRI Reflex NeuroIntegration Therapy. Int J Phys Med Rehabil 5: 6. [Ref.]
- Bell C, Whitney J, Deiss T, Tatarinova Т, Franckle L, et al. (2019) The Effect of the MNRI Method on Neurotransmitter Biomarkers of Individuals with Neurodevelopmental Disorders. Neurosci Med 10: 292-321. [Ref.]
- Bradley CL, Damiano DL (2019) Effects of Dopamine on Motor Recovery and Training in Adults and Children with Nonprogressive Neurological Injuries: A Systematic Review. Neurorehabil Neural Repair 33: 331-344. [Ref.]
- Drobyshevsky A, Takada SH, Luo K, Derrick M, Yu L, et al. (2015) Elevated Spinal Monoamine Neurotransmitters after Antenatal Hypoxia-Ischemia in Rabbit Cerebral Palsy Model. J Neurochem 132: 394-402. [Ref.]
- Schaffer S, Kim HW (2018) Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol Ther (Seoul) 26: 225-241. [Ref.]
- Shoulders MD, Raines RT, Annu Re V, Biochem (2009) 78, 929--958. (b) Fields, GB. Org Biomol Chem. 2010; 8: 1237-1258.
- Sheridan KJ (2009) Osteoporosis in adults with cerebral palsy. Dev Med Child Neurol 51 Suppl 4: 38-51. [Ref.]
- Scassellati C, Bonvicini C, Faraone SV, Gennarelli M (2012) Biomarkers and Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analyses J Am Acad Child Adolesc Psychiatry 51: 1003- 1019.e20. [Ref.]
- Irsfeld M, Spadafore M, Prüß BM (2013) β-phenylethylamine, a small molecule with a large impact. Webmedcentral 4: 4409. [Ref.]
- Masgutova SK, Akhmatova NK, Sadowska L, Shackleford P, Akhmatov EA (2016) Neurosensorimotor Reflex Integration for Autism: a New Therapy Modality Paradigm. J Pediatr Neurol Disord 2: 107. [Ref.]
- Nowak K, Sendrowski K (2016) Neurophysiological Aspects of NeuroTactile Therapy of Masgutova Neurosensory Motor Reflex Integration MNRI® Method. Med Rehabil 20: 1-10. [Ref.]
- Akhmatova N, Akhmatova E, Lebedinskaya O (2016) MNRI: An immunomodulating effect on lymphocyte subpopulation structure of children with Down syndrome. Russ J Immunol 10: 485-487.
- Akhmatova E, Akhmatova N, Masgutova S (2016) MNRI: Immunocorrective Effect on the Immune Status of Children with Down Syndrome. 17th Biennial Meeting of the Euopean.
- Ortego L, Pelican E, Callaba L, Marks T (2015) An investigation of the effects of MNRI techniques on the educational performance of kindergarten students. Svetlana Masgutova Educational Institute: Orlando, FL, USA. [Ref.]
- Masgutova, Regner A (2008) Language development using sensorymotor integration approach. Scientific edition: Prof T Galkowski, Dr B Dolyk.
- Koberda JL, Moses A, Koberda P, Koberda L (2013) Clinical Advantages of Quantitative Electroencephalogram (QEEG)-electrical Neuroimaging Application in General Neurology Practice. Clin EEG Neurosci 44: 273-285. [Ref.]
- Shin YK, Lee DR, Hwang HJ, You SJH, Im CH (2012) A Novel EEGbased Brain Mapping to Determine Cortical Activation Patterns in Normal Children and Children with Cerebral Palsy during Motor Imagery Tasks. NeuroRehabilitation 31: 349-355. [Ref.]
Download Provisional PDF Here
Article Type: REVIEW ARTICLE
Citation: Masgutova S, Koberda JL, Shackleford P, Nowak K, Akhmatova N, et al. (2020) Effect of the MNRI Reflex Neuromodulation on the QEEG and Neurotransmitters of Children Diagnosed With Cerebral Palsy. J Neurol Neurobiol 6(3): dx.doi.org/10.16966/2379-7150.170
Copyright: © Masgutova S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
SCI FORSCHEN JOURNALS
All Sci Forschen Journals are Open Access
