Abstract
Background: Induced mild hypothermia (MIH) is generally an effective intervention for reducing intracranial pressure (ICP) in patients with severe traumatic brain injury (TBI). Unfortunately, MIH also influences brain tissue oxygenation (PbtO2). The significance of the PbtO2 change is unknown. We report a patient with severe traumatic brain injury (TBI) with refractory intracranial hypertension requiring MIH which resulted in a decrease in his PbtO2 values.
Case report: The patient presented in a coma following an altercation. He was found to have a large left subdural hemorrhage with significant mass effect requiring craniectomy. He subsequently developed refractory intracranial hypertension requiring medically induced coma and MIH. As temperature decreased, his PbtO2 values decreased from 23-35 mmHg to 6-10 mmHg. There was a significant reduction in PbtO2 values as temperature decreased (p<0.001). ICP did not change with a decrease in temperature and remained elevated (p=0.78).
Conclusion: PbtO2 significantly decreased linearly with the decrease in body temperature. Future studies should evaluate the clinical significance of the reduction in PbtO2 in patients with severe TBI and refractory intracranial hypertension requiring MIH. Recognizing expected changes in PbtO2 monitoring with MIH is important for managing neurocritically ill patients.
Keywords
Mild hypothermia; Intracranial pressure; Brain tissue oxygenation (PbtO2); Traumatic brain injury
Introduction
Therapeutic temperature modulation with induced mild hypothermia (MIH) exerts neuroprotection and attenuates secondary cerebral insults after traumatic brain injury (TBI) [1]. MIH is effective in reducing elevated intracranial pressure (ICP) in TBI [1]. In addition to controlling intracranial hypertension, optimizing partial brain oxygen tension (PbtO2 ) may also decrease morbidity in TBI patients [2]. PbtO2 is often used in conjunction with monitoring ICP and brain temperature to tailor hemodynamic management in patients with acute traumatic brain injuries [3]. Normal PbtO2 values for brain tissue oxygen varies from 25 to 35 mmHg. Values lower than <20 mmHg indicate inadequate brain tissue oxygenation and perfusion [3]. Risk of morbidity and mortality in TBI has shown to significantly increase with values <15 mmHg [3]. Monitoring cerebral oxygenation, perfusion, and brain temperature may identify changes associated with neurological worsening prior to changes in examination [3]. Induced mild hypothermia inhibits multiple pathological processes simultaneously that may attenuate secondary biochemical cascades that are activated after acute ischemic injury through reduction of metabolism, perfusion, and edema. Also, reduction of ICP by MIH may help improve overall perfusion.
However, the significance of PbtO2 monitoring with MIH is largely unknown. In this study, we have investigated the effects of MIH on concomitantly obtained indices of PbtO2 and ICP in a patient with severe TBI. In our study, PbtO2 decreased linearly with the decrease in body temperature. There was a significant reduction in PbtO2 values as temperature decreased (p<0.001). PbtO2 reduction was evident even though the ICP did not change with a decrease in temperature and remained elevated (p=0.78), a finding that has not been reported previously. This finding raises concerns about the effectiveness of MIH in lowering ICP in patients with severe TBI who are already in a medically-induced coma.
Case Report
A 23 years old male was brought to the emergency department in an unresponsive condition after sustaining a traumatic head injury. His Glasgow coma scale (GCS) score was 3 (E: 1, V: 1, M: 1) requiring intubation. Vital signs in the emergency department were notable for a blood pressure of 113/76 mmHg, respiratory rate of 14 per min, heart rate of 47 bpm and temperature of 36.2°C. On physical examination, he had a dilated fixed left pupil, a reactive right pupil, and flaccid quadriplegia. His labs were significant for acute kidney injury with a creatinine of 1.24mg/dl. There was no coagulopathy. Computed tomography (CT) of the head showed a left hemispheric subdural hematoma measuring 8 mm in thickness and a left to right midline shift of 12 mm (Figure 1). He was intubated and immediately taken to the operating room for left fronto-parieto-temporal craniectomy with the evacuation of the subdural hematoma. External ventricular drain (EVD) was placed on the right at Kocher’s point. ICP was 20 mmHg. Hyperosmolar therapy was continued to maintain ICP of less than 20 mmHg. PbtO2 monitoring (Licox, Integra, Plainsboro, NJ, USA) was placed on the right frontal area approximately 2 cm anterior to the EVD placement and 3 cm to the right of midline. Two days later, he developed refractory intracranial hypertension despite osmotic therapy. Pentobarbital coma was induced targeted for burst suppression. Despite medically induced coma, his ICPs remained elevated. He was then placed on MIH protocol using an advanced surface cooling device (Arctic Sun, Bard Medical, Covington, GA, USA). Body temperature was reduced from 36.4°C to target 32 to 34°C. Patient’s temperatures achieved ranged from 31.7°C to 33.8°C over next 2 days. Patient’s ICP, brain temperature, and PbtO2 were continuously monitored. ICP initially improved but the reduction was not sustained, and it remained elevated (95% confidence interval -1.39 to 1.84 mmHg; R2 0.0026; p=0.78). There was a significant linear reduction in PbtO2 with a decrease in temperature (95% confidence interval 2.46 to 6.06 mmHg; R2 0.44; p<0.001) with a marked reduction below 34°C (Figure 2A, 2B and 2C). Data analyzed with Graph Pad 7.0 (LaJolla, CA, USA).
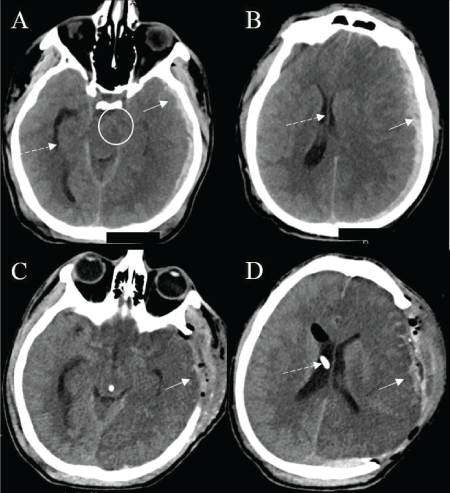
Figure 1: Computed tomography (CT) of the head. CT scan showed a left hemispheric subdural hematoma (SDH) measuring 8 mm in thickness (A and B, solid arrow) and a left to right midline shift of 12 mm (B, dashed arrow) and uncal herniation (A, circle). Postoperatively, CT head shows evacuation of the SDH (C and D, solid arrow). There is an external ventricular device (EVD) terminating in the right lateral ventricle (D, dashed arrow). Note the large, evolving hypodensity on the left hemisphere (D).
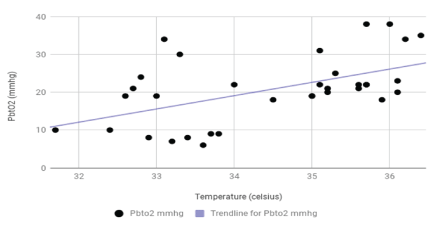
Figure 2A: Graph of Changes in PbtO2 with Changes in Temperature. As the temperature decreased, there was a decrease in PbtO2 (Cross markers; R2 =0.44).
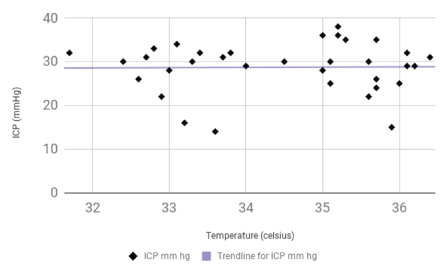
Figure 2B: Graph of Changes in ICP with Changes in Temperature. ICP remained relatively unchanged with decrease in temperature (R2=0.0026).
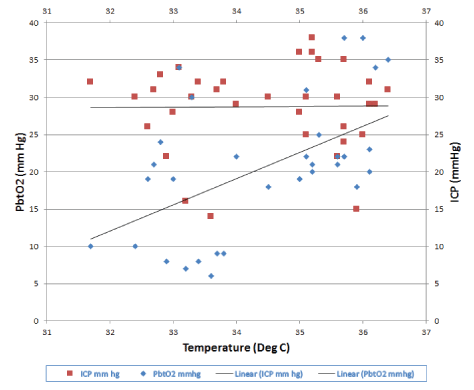
Figure 2C: Graph of changes in Pbto2 and Icp with changes in temperature. ICP remained relatively unchanged with decrease in temperature (R2 =0.0026).
Patients continued to deteriorate with the development of multisystem organ failure. After multi-disciplinary discussions, the family requested transition to comfort care.
Discussion
MIH is generally an effective intervention in reducing intracranial pressure but can also affect cerebral blood flow and PbtO2 [4]. In our study, we observed a correlation between PbtO2 with a decrease in temperature most obvious at temperatures below 34°C. The reduction in PbtO2 can be explained by several physiological factors:
- A decrease in body temperature below 37°C is associated with decreased oxygen utilization in the brain [4].
- Hypothermia can cause a leftward shift in oxygen distribution curve [5]. This enhances the affinity of oxygen to hemoglobin causing reduced oxygen release and reduced availability to brain cells [5].
- The rate of oxygen diffusion is directly proportional to the tension gradient between the vessels and the mitochondria, the conductance through the tissue, and the time available for diffusion [6]. A mismatch in oxygen metabolism/delivery following TBI can be contributed to the microvascular injury, increased diffusion distances following cerebral edema, and mitochondrial alterations secondary to membrane damage [7].
All the above factors can contribute to decrease oxygen availability, impaired diffusion, and eventual tissue hypoxia.
Studies have investigated the direct effect of hypothermia on PbtO2 . In a study of 30 patients cooled to a minimum of 33°C. Gupta et al. [8] investigated the effects of decreasing brain temperature on indices of brain parenchymal oxygenation and metabolism in patients with severe head injury [8]. They found no significant change in arterial O2 , CO2 , and pH as brain temperature was reduced to 35°C. However, below 35°C, there was a significant reduction in PbtO2 , which occurred despite an increase in PaO2 [8]. A retrospective analysis by Flynn et al. [2] studied the effects of therapeutic hypothermia on intracranial pressure and partial brain oxygen tension in patients with severe traumatic brain injury. ICP and PbtO2 were measured using the Licox system and core temperature was recorded through rectal thermometer [2]. Data were analyzed at the hour before cooling, the first hour at the target temperature, two consecutive hours at the target temperature, and after 6 hours of hypothermia [2]. A mean decrease in ICP of 4.3 ± 1.6mmHg from pre-cooling to the first epoch of hypothermia was observed [2]. There was also a mean decrease in PbtO2 of 7.8 ± 3.1mmHg from pre-cooling to stable hypothermia [2]. In our patient, we observed a decrease of about 30 mmHg in PbtO2 with a 5 degree Celsius decrease in temperature. The hypothermia protocol was activated for duration of 2 days. The body temperature was reduced from 36.4 to 31.7°C over two days. Graph 2c also represents changes in ICP and PbtO2 over time. Small microtrauma can possibly occur with licox implantation, however no such variations demonstrating local injury were noted in PbtO2 readings in our patient. CT head following implantation showed no focal hemorrhage or sign of local tissue damage. The patient developed multiorgan failure over subsequent days following initiation of hypothermia therapy. The creatinine value, which was 1.2 mg/dl at initial presentation deteriorated to 4.88 mg/ dl. Both PbtO2 and ICP values responded well to hypothermia therapy during the initial phase. However, the possibility of organ failure and decline in tissue oxygenation cannot be ignored given the critical condition of the patient during later half of hypothermia therapy.
If hypothermia consistently decreased tissue oxygenation, it has the potential of causing cerebral ischemia [9]. Unlike many pharmacological treatments that tend to antagonize a single neurochemical process, hypothermia inhibits multiple pathological processes simultaneously [10]. Hypothermia may attenuate secondary biochemical cascades that are activated after acute ischemic injury through reduction of metabolism, perfusion, and edema [10, 11]. On the other hand, reduction of ICP caused by MIH may help improve perfusion. Compared to a head cooling mechanism whole body cooling appears a more suitable system as it was shown that RhinoChill, for example, has effects like epistaxis, cold-related tissue damage, emphysema, hypertension, hypoxia, and the probe placement can be troublesome in critically ill patients. While early studies suggested a reduction in mortality and improved neurological function after TBI after 48 hours of MIH [12]. The BrainHypothermia trial showed no improvement in neurological outcomes or risk of mortality in severe TBI patients compared to patients who were maintained normothermic [13]. In our case, MIH failed to induce any significant reduction in ICP. ICP remained elevated throughout the entire course of hypothermia protocol, which raises concerns about the effectiveness of MIH in lowering ICP in patients with severe TBI who are already in a medically-induced coma.
Licox system can have some discrepancies in monitoring PbtO2 , more pronounced at extremes of temperatures. The effect of temperature ranges on the accuracy of measurement of PbtO2 has also been studied. The difference in measurements of PbtO2 , temperature, and ICP can be observed with different devices used for monitoring. A study to assess accuracy and response time of PbtO2 probe and the automated card calibration system over a broad range of PbtO2 values and temperature reported that the probe is overall accurate but may under-estimate oxygen tension at a higher temperature and vice versa [14]. Licox PMO probe has a small tendency to underestimate oxygen tension (mean error, -3.8 ± 3.5%) that is more pronounced between the temperatures of 33 and 39°C [14]. The thermistor of the PMO probe also has a tendency to underestimate temperature when compared with a resistance thermometer (mean error, -0.67 ± 0.22°C) [14]. The card calibrated system was also found to introduce some variability in measurement of oxygen tension when compared with a manually calibrated system [14]. These variations can cause discrepancies when the PbtO2 probe is used to guide or in conjunction with temperature directed treatment strategies, such as what we have reported.
Conclusion
Our case demonstrates that brain parenchymal oxygenation decreases with a decrease in body temperature. Ensuring adequate cerebral perfusion is of fundamental importance in managing patients with severe TBI. Monitoring brain tissue oxygen tension is becoming more commonly used in conjunction with ICP monitoring in severe TBI. In those patients requiring MIH for control management of refractory intracranial hypertension, it is unknown how robust PbtO2 values remain. We showed that there is a decrease in PbtO2 with decreasing temperature even if ICP remains refractory. Future studies should evaluate the clinical significance of this observation.
Article Information
Article Type: CASE REPORT
Citation: Katyal N, Newey CR (2018) Linear Decrease in Brain Tissue Oxygenation (PbtO2 ) with Decreasing Body Temperature during Induce Hypothermia. J Neurol Neurobiol 4(1): dx.doi.org/10.16966/2379- 7150.144
Copyright: © 2018 Katyal N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 15 Dec, 2017
Accepted date: 12 Jan, 2018
Published date: 18 Jan, 2018





