Abstract
Milk fat globule epidermal growth factor 8 (MFG-E8) plays an important role in the regulation of neuroinflammation. Studies have indicated it has altered expression in some neurodegenerative diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD). Progressive supranuclear palsy (PSP) is a neurodegenerative disorder in which tau aggregates in neurons and glia. So far, little is known about the underlying mechanisms of this disorder. To study whether MFG-E8 may play a role in the pathology of PSP, we carried out an immunohistochemical study on autopsy brain tissue from 6 PSP patients and 6 matched controls. We found MFG-E8 expression was significantly increased in neurons of the substantia nigra (SN) (P<0.01), pons (P=0.02) and medulla (P=0.04) of the PSP cases. However, no obvious changes of its expression were found in the frontal lobe, temporal lobe, caudate, putamen, or cerebellum (P>0.05). The findings demonstrate that MFG-E8 may be partially associated with the pathogenesis of PSP. Further research focused on MFG-E8 may provide new insights into novel therapies for PSP.
Keywords:
Alzheimer’s disease; Parkinson’s disease; Substantia nigra; Pons; Medulla
Introduction
Milk fat globule epidermal growth factor 8 (MFG-E8) is a glycoprotein secreted by different cell types. It is involved in the regulation of central nervous system homeostasis through inhibiting immune responses [1-3]. It is reported to exert an important role in neurodegenerative diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD) [4,5]. It has been shown to alleviate AD pathology through clearance of amyloid β (Aβ) plaques [4]. In PD, enhanced expression of MFG-E8 is observed. However in vivo experiments indicate it does not take part in nigrostriatal injury [5]. This indicates MFG-E8 might have different effects in different neurodegenerative diseases.
Progressive supranuclear palsy (PSP), is characterized by increasing intracellular formation of misfolded tau protein. There is preferential involvement of the substantia nigra (SN), pontine tegmentum, oculomotor nucleus, and medulla. While tau-positive neurofibrillary tangles (NFTs), coiled bodies, threads, and tufted astrocytes have been shown [6], the underlying mechanisms are still unclear. We analyzed the expression of MFG-E8 in brain tissues of PSP and normal patients to investigate whether it has any relationship with PSP pathology
Methods
Human brain samples
Brain samples were obtained shortly after death and then stored in the human brain bank of the Kinsmen Laboratory of Neurological Research. Diagnosis of the control and PSP cases had all been carefully confirmed by certified neuropathologists.
A total of 6 cases with PSP were evaluated. The PSP cases were all of the Richardson type with the confirmative upward gaze palsy and lack of response to dopaminergic stimulation. They each suffered from a profound movement disorder. They were all cognitively alert showing no evidence of dementia. Case 1 was a 62 year old male with a 7 year history of progressive PSP. He was unresponsive to all forms of therapy including dopaminergic stimulation and was terminally immobile. Case 2 was a 68 year old female presenting with instability. She improved in hospital upon termination of Sinemet therapy but expired at home 17 months later. Case 3 was a 66 year old woman with a 6 year history of progressive PSP. She was unresponsive to Lisuride therapy and was terminally immobile. Case 4 was a 75 year old female with a progressive movement disorder that did not respond to Sinemet leading to the conclusion she was suffering from PSP. She had previously been PET (positron emission tomography) scanned verifying changes consistent with a movement disorder. She had trouble swallowing and suddenly expired. Case 5 was a 73 year old male with progressive motor impairment causing frequent falls. He showed absolutely no improvement on 8 pills a day of Sinemet. He was confined to a wheelchair and expired in a nursing home. Case 6 was a 64 year old male who suffered from advanced immobility. He was tried on Sinemet which was later discontinued as being ineffective. He expired following 18 months in a hospital extended care unit.
These cases were compared with 6 controls. Control cases were selected from those dying of causes unrelated to any neurological disorder and where post mortem study verified no involvement of the CNS. Briefly, Case 1 was a 78 year old male who died from sudden cardiac arrest. Case 2 was an 80 year old male who died suddenly from chronic obstructive pulmonary disease. Case 3 was a 72 year old male who died from lung cancer. Case 4 was a 65 year old female who died from a thyroid cancer. Case 5 was an 80 year old female who died from bronchogenic carcinoma. Case 6 was 69 year old female who died of cardiac arrest.
Post mortem delay did not differ significantly between the PSP and control group. The frontal lobe, temporal lobe, caudate, putamen, substantia nigra, pons, medulla and cerebellum were selected for study. The areas were cut into blocks of about 5 mm thickness, and then immersed for 2-3 days in 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) at 4°C. They were then transferred to a maintenance solution of 15% sucrose in 0.1 M PBS. Sections were cut at 30 μm thickness, collected and stored in the maintenance solution before use. Comparative details of the age and sex of controls and PSP cases are given in table 1.
|
Age |
Sex |
Control |
78 |
M |
Control |
80 |
M |
Control |
72 |
M |
Control |
72 |
F |
Control |
80 |
F |
Control |
67 |
F |
PSP |
62 |
M |
PSP |
68 |
F |
PSP |
66 |
F |
PSP |
75 |
F |
PSP |
73 |
M |
PSP |
64 |
M |
Table 1: Summary of cases examined in this study
Age (Mean ± SEM): Control: 74.83 ± 2.17 and PSP: 68 ± 2.08. One-way ANOVA was carried out to test significance. * P<0.05 for control group compared with PSP group.
Immunohistochemistry
Immunostaining for MFG-E8 (Figure 1) was carried out using a specific polyclonal antibody (Abnova, Taiwan). Immunostaining for HLA-DR (Figure 2), a marker for neuroinflammation, was carried out using the human anti-HLA-DR mouse monoclonal antibody CR3/43 (DakoCytomation).
Immunostaining was done using previously described protocols [7]. Briefly, sections were pretreated with 1.5% H2 O2 in PBS for 15 min, and then non-specific protein interference blocked by treatment for 1 h in a mixture of a 1/50 dilution of 5% skim milk and diluted normal rabbit serum. They were then incubated overnight at room temperature with the mouse polyclonal MFG-E8 antibody (1:1000 dilution; Abnova, Taiwan) or the mouse monoclonal CR3/43 ( DakoCytomation 1:1000) mixed with 60 μl of a 1/50 dilution of normal rabbit serum. The sections were next treated with a biotinylated rabbit anti-mouse antibody (1:2000 dilution; DakoCytomation) for 1 h at room temperature, followed by treatment for 1 h with the Vector ABC kit (Vector, US) according to the manufacturer’s directions. Peroxidase labeling was visualized after incubation in 0.01% 3,3-diaminobenzidene containing 1% nickel ammonium sulfate, 50 mM imidazole and 0.001% H2 O2 in 0.05 M Tris-HCl buffer. When a dark purple color developed, the reaction was terminated by washing the sections in PBS. They were then dried and cover slipped. Images were obtained using an Olympus BX51microscope under constant and predefined light and exposure settings with Image Pro software (Improvision Inc., Waltham, US).
MFG-E8 evaluation
MFG-E8 expression on the sections was considered immunopositive if the reaction product was present within a cell. An immunohistochemical score (IHS) method was used in this study. Briefly, the IHS combined a quantification of immunoreactive cells (quantity score) with an intensity score (staining intensity score). For evaluation of MFG-E8 positive cells, 5 random images at 10 × object magnification (0.567 mm2 ) were acquired from the stained sections of each region. For quantification, images were transformed into 8 bit mode with Image J 1.49 (National Institute of Health, Bethesda, US). The threshold levels were then adjusted to allow the separation of MFG-E8 positive cells from non-stained cells. All particles between 100-500 pixel size were counted using the “analyzed particles” command. The number of particles per image was acquired for the quantity score. No cell staining was scored as 0, 0-25% of cells stained as 1, 26-50% as 2, 51-75% as 3, 76-100% as 4. The intensity of MFG-E8 immunoreactive cells was determined by visual observation to get the intensity score. All these were done for each case individually. Intensity score for no staining was 0; 1, faint cytoplasmic staining without background staining; 2, moderate cytoplasmic staining; 3, strong cytoplasmic staining. The IHS was calculated by multiplying the quantity and intensity scores.
Statistics
All values are expressed as mean ± standard error of the mean (S.E.M.). Comparisons were made with Mann-Whitney U test using SPSS 19.0 software. The significance was established at a level of p<0.05.
Results
Comparison of MFG-E8 expression between normal and PSP patients
We studied the brain regions described in Methods 2.1 from 6 control and 6 PSP patients by immunohistochemistry. MFG-E8 was detected in glial, neuronal and epithelial cells of all brain regions studied. In the frontal lobe, temporal lobe, caudate, putamen and cerebellum of both groups, we only found faint to moderate cytoplasmic MFG-E8 expression. And in these areas there were no significant differences between PSP and normal cases (P>0.05) (Table 2). However in the SN (P<0.01), pons (P=0.02) and medulla (P=0.04), there was a substantially higher MFG-E8 expression (Table 2). Additional details can be found in supplemental table 1.
Regions |
Control |
PSP |
P value |
Frontal |
1.17 ± 0.31 |
1.33 ± 0.50 |
0.2 |
Temporal |
1.50 ± 0.62 |
0.50 ± 0.22 |
0.8 |
*SN |
1.00 ± 0.37 |
4.50 ± 1.09 |
<0.01* |
Caudate |
0.83 ± 0.31 |
1.67 ± 0.56 |
0.24 |
Putamen |
0.67 ± 0.33 |
1.33 ± 0.21 |
0.07 |
*Pons |
2.17 ± 0.48 |
5.83 ± 1.17 |
0.02* |
*Medulla |
1.67 ± 0.33 |
1.83 ± 0.31 |
0.04* |
Cerebellum |
1.33 ± 0.33 |
1.17 ± 0.40 |
0.79 |
Table 2: Immunohistocemical Score of MFG-E8 immunostaining in brain regions of PSP patients and normal controls
Immunohistochemical score=quantity score × intensity score; *P<0.05.; SN, Substantia nigra; PSP, progressive supranuclear palsy.
In the SN of PSP cases, there was an obvious reduction in pigmented dopaminergic neurons accompanied by extracellular neuromelanin from lost neurons (Figures 1C and 1D). The SN of control cases was normal (Figure 1A and 1B). Stronger MFG-E8 expression was observed in the residual melanised neurons of PSP. In the SN region of PSP cases there was also a severe depletion of neurons with an increased expression of MFG-E8 (Figure 1G and 1H), compared with control cases (Figure 1E and 1F). In the medulla of PSP cases (Figure 1K and 1L), we observed a stronger signal of MFG-E8 in the tegmentum. Only weak expression was seen in the inferior olivary nucleus.
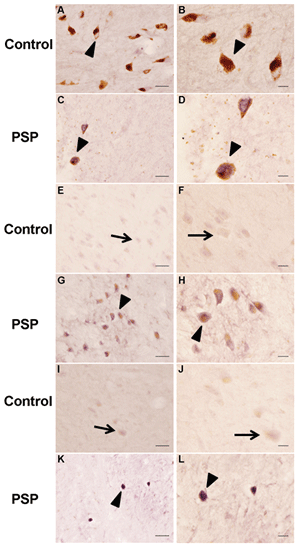
Figure 1: MFG-E8 immunostaining in brains from SN (A, B, C, D), pons (E, F, G, H) and medulla (I, J, K, L) of normal and PSP subjects. Strong MFG-E8 immunostaining was observed in the remaining neurons of the SN (C, D), pons (G, H), and medulla (K, L) of typical PSP cases. Scale bars=50 μm (A, C, E, G, I, K) and 20 μm (B, D, F, H, J, L).
Figure 2 illustrates comparative immunostaining for HLA-DR of the pedunculopontine area in PSP and control cases. The antibody CR3/43 is a well established marker for this microglial protein. Inflammation results in a sharp up-regulation of activated microglia as shown in Figure 2A for PSP case 1 (female, age 75) and 2B for PSP case 2 (male, age 64). Figure 2C for control case 1 (male, age 78) and 2D for control case 2 (female, age 80) provide comparisons of typical resting microglial immunostaining. All six PSP cases and all six controls showed immunostaining indistinguishable from the examples illustrated. These data show that upregulation of MFG-E8 expression coincides with inflammation in PSP cases.
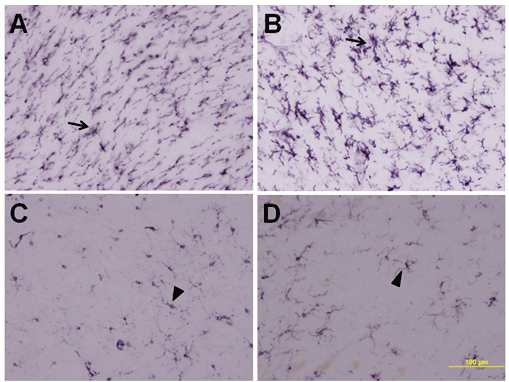
Figure 2: CR3/43 immunostaining of the pons in typical PSP and control cases. A, PSP case 2; B, PSP case 4; C, control case 4; D, control case 6. Notice the intense immunostaining of activated microglia in the PSP cases compared with controls. Scale bar=100 μm.
Discussion
In the present study, we demonstrated for the first time expression of MFG-E8 in PSP brains. It was expressed to some degree in all the brain regions that were studied. Widespread expression is consistent with its previously reported inhibitory role in neuroinflammation [1,8]. MFG-E8 was initially described as a mammary epithelial cell surface protein with unique anti-inflammatory effects [9]. More recently it has been identified as a glycoprotein involved in the pathology of some neurodegenerative diseases [4,5]. For example, in AD cases there was decreased overall MFG-E8 expression which was associated with senile plaque formation [4]. This suggests a possible role in alleviating the pathology. There was an increased expression of this protein in the SN of PD, although no regulatory role was identified. This raised the possibility of different roles of MFG-E8 in different neurodegenerative diseases.
Thus we undertook a study to examine its role in PSP. We selected a series of sections from different regions of PSP and control brains and showed that the SN, pons and medulla had a stronger expression of MFG-E8 in the PSP sections. This suggests an involvement in the pathology. However, no obvious changes in expression were seen in the frontal lobe, temporal lobe, caudate, putamen and cerebellum regions. Since the PSP and control brains were matched, these differences cannot be attributed to gender and postmortem degradation. There was a slight difference in age (68 in PSP cases compared with 74 in controls) but age has not been identified as a contributing factor in MFG-EB expression.
We speculate that the low level of expression of MFG-E8 detected in control tissues is consistent with a standby function and ability to respond quickly to immune stimuli. Once activated, it would be able to modulate the immune response. However, the role of MFG-E8 could vary in different brain regions.
We found that in PSP, MFG-E8 was co localized with remaining neurons in areas of widespread neuronal loss. So it is possible that MFG-E8 has a protective effect on these neuronal cells. This could result in inhibition of production of inflammatory cytokines such as TNF-α, IL-1β, inducing such a protective effect on the neuronal cells.
Upregulation of MFG-E8 is known to occur in affected areas of Alzheimer disease and Parkinson disease brains. They have completely different pathologies from the tauopathy which underlies PSP. The common thread in all these diseases is a reaction to neuroinflammation. In PSP, surviving neurons in affected areas strongly express MFG-E8 suggesting an important neuronal protective role.
Further research focused on MFG-E8 and its protective potential for neurons under attack may provide new insights into novel therapies for PSP and perhaps other neurodegenerative disorders.
Acknowledgements
This research was supported by grants from the Plan of Science and Technology of Guangzhou, China (Grant No. 1561000166) and contributions from individual British Columbians.
Article Information
Article Type: Research Article
Citation: Shi X, Yu S, McGeer E, McGeer PL (2016) Milk Fat Globule Epidermal Growth Factor 8 (MFG-E8) Expression in Progressive Supranuclear Palsy. J Neurol Neurobiol 2(5): doi http://dx.doi.org/10.16966/2379-7150.131
Copyright: © 2016 Shi X, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 01 Oct 2016
Accepted date: 24 Oct 2016
Published date: 28 Oct 2016



