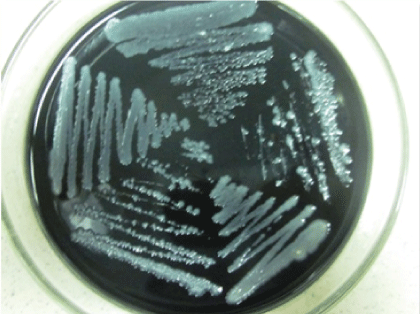
Figure 1: Campylobacter colonies on CCDA plates


Shakila Sultana*
Department of Microbiology, Primeasia University, Dhaka, Bangladesh*Corresponding author: Shakila Sultana, Department of Microbiology, Primeasia University, 12, Kemal Ataturk Avenue, Banani, Dhaka-1213, Bangladesh, E-mail: sultanashakila130@gmail.com
Poultry meat is frequently contaminated with Campylobacter strains and is thought to be the major source of organisms causing human Campylobacter gastroenteritis. This study was carried out to detect Campylobacter spp. in raw chilled chicken meat, chicken gut, pre-cooked frozen chicken nuggets and chicken sausages from super markets in different areas of Dhaka city, Bangladesh. Isolation of Campylobacter spp. was done using the conventional method in which 10g of each sample (except chicken gut) was homogenized with 90ml of enrichment broth and incubated in microaerophilic condition at 42°C for 48hours. Samples were then streaked onto Campylobacter charcoal differential agar base supplemented with CCDA selective supplement and was incubated in microaerophilic condition at 42°C for 48h. For chicken gut, swabs are taken from the inner walls of the gut and directly streaked on CCDA plates. Microscopic examination, gram staining, biochemical tests were done for the presumptive identification of Campylobacter spp. The results showed that Campylobacter spp. can be found in various parts of raw chicken meat, chicken sausages, and chicken gut while chicken nuggets showed the absence of Campylobacter spp. This finding reflects the risk of cross-contamination while handling of poultry in our kitchen during the preparation of food.
Food safety; Food hygiene; Campylobacteriosis; Poultry; Poultry products
Human enteritis in developed and developing countries is mostly caused by Campylobacter spp. with C. jejuni, the most important pathogenic strains belonging the thermotolerant group, considered as the leading cause for foodborne and waterborne gastroenteritis worldwide [1]. Campylobacter jejuni is most often implicated as the causative agent of Campylobacteriosis and are commonly isolated from chicken and cattle, and chicken is expected to be one of the major sources of infection.
Poultry meat and poultry products consumption and production show an upward trend worldwide for a number of reasons including its increased use in food preparations, strong consumer preference, and a great demand on a rich protein source (20–23%) with reasonable cost [2-4].
Campylobacter occurs as a commensal microorganism in the microflora of the gastrointestinal tract of poultry, thus it is likely to occur on the exterior of the bird as well as from the contamination of the growth environment. The normal body temperature of poultry encourages the gastrointestinal establishment of this organism that supports their high optimal growth temperature [5]. It was reported that Campylobacter infections in human are usually associated with the ingestion of improperly handled or undercooked food, mainly poultry products [6].
As Campylobacter occurs as natural microbial flora in the gastrointestinal tract of poultry, after slaughtering it is possible that they can spread to poultry carcasses, parts and products. Moreover, C. jejuni survives well in modified atmosphere and vacuum packaging and also under refrigerated temperatures [5] making it plausible to occur in packaged chicken products that have gone through poor handling and processing. The prevalence of Campylobacter spp. on raw poultry products ranges from 0 to 100%, with an average of 62%.
The aim of this study was to evaluate the prevalence of Campylobacter spp. in raw chicken meat (chilled), chicken gut and packaged chicken-based products that have been stored in freezing condition.
At slaughter: One chicken gut was collected from the kitchen in a sterile plastic bag and submitted to the laboratory as soon as possible.
At super market: Three intact packets of different brands of frozen pre-cooked chicken nuggets and chicken sausages were bought from the supermarket and submitted to the laboratory. Raw chilled minced chicken meat was bought and submitted intact to the laboratory in a plastic bag. 3
Campylobacter Enrichment Broth: 25g was thoroughly mixed in 1liter water. It was swirled and autoclaved 15 min at 121°C (in screw-capped bottles).
For Chicken nuggets, Chicken Sausages and Minced Chicken Meat: 10 gram of weighed sample was homogenized with 90 ml of Campylobacter enrichment broth and incubated for 24 hours at microaerophilic condition at 42°C. After that streaking was done on CCDA (Campylobacter Charcoal Differential Agar) this is selective for Campylobacter spp. and incubated at microaerophilic condition at 42°C for 48 hours. Plates were observed. For chicken gut, cotton swabs are taken from inner walls of the colon and directly streaked on CCDA plates.
Reagents used are listed in Appendix I and II.
Plates were observed after 48 hours of incubation (Figure 1).

Figure 1: Campylobacter colonies on CCDA plates
For chicken sausages and raw chilled minced meat, heavy to moderate growth was found. The colonies were gray in color, glistening, spreading to round shaped, elevated and the texture was creamy. For chicken nuggets samples, no growth was found.
Material from a suspected colony was suspended in saline and evaluated by light microscope for the characteristic. Spiral or curved slender rods with a corkscrew-like motility were observed though not very clearly. Older cultures showed less motile coccoid forms.
Gram staining was performed from suspected colonies. All were gram negative.
The oxidase reagent (tetramethyl-p-phenylenediamine dihydrochloride) was taken on a filter paper and placed in a Petri dish. The filter paper was moistened with distilled water. The colony from the CCDA agar was picked with help of a tooth pick and soaked on the oxidase solution region. The appearance of the blue color within 10 seconds would indicate a positive result.
A small amount of growth (single, discrete colony) from CCDA plate was placed on a clean microscope slide. A few drops of H2O2 was added to the smear. It was mixed with a toothpick. A positive result was indicated by the rapid evolution of O2 as evidenced by bubbling.
This test is used to determine whether a microorganism, by the action of the enzyme hippurate hydrolase, can hydrolyze sodium hippurate to benzoic acid and glycine. 0.4 ml of sodium hippurate solution was inoculated with heavy inoculums (single colony). It was shaken by vortex. Incubated for 2 hours at 37°C aerobically. 0.2 ml of Ninhydrin solution was added slowly down the side to form an overlay. It cannot be shaken. Tubes reincubated at 37°C and checked for color change after 10 to 20 minutes.
Presence of a deep blue or purple color indicates a positive reaction. The tubes that were colorless showed negative for hippurate hydrolysis test (Table 1).
Sample |
Growth at 42°C |
Oxidase test |
Catalase test |
Hippurate hydrolysis test |
Chicken Nuggets |
||||
Sample 1 |
- | - | - | - |
Sample 2 |
- | - | - | - |
Sample 3 |
- | - | - | - |
Chicken Sausages |
||||
Sample 1 |
+ | + | + | + |
Sample 2 |
+ | + | + | + |
Sample 3 |
+ | + | + | + |
Raw Chilled Minced chicken meat |
+ | + | + | - |
Chicken Gut |
+ | + | + | + |
Table 1: Biochemical test results of the colony found on CCDA plate
In this study, Campylobacter spp. was isolated from poultry and poultry products. Enrichment of chicken nugget samples, chicken sausage samples and chilled raw minced meat samples was performed prior to isolation because organisms might be under stress due to refrigeration. In case of the chicken gut, cotton swabs were taken from the inner walls of the gut (as they are microaerophilic, cotton swab stick was pushed inside) and streaked directly on selective media. Results showed that chicken sausages, chilled raw minced meat and chicken gut contained Campylobacter spp. Campylobacter was absent in chicken nugget samples. All the incubations were done at 42°C as most of the pathogens cannot grow at this temperature and thus the contamination risk is low here.
Presence of Campylobacter spp. in pre-cooked frozen poultry products (chicken sausages) indicates improper handling. Due to the fact that Campylobacter survives well in modified atmosphere and vacuum packaging and also under refrigerated temperatures, there are possibilities that it could occur in packaged chicken products that have gone through poor handling and processing. Consumption of insufficiently cooked frozen poultry products might be the cause of acquiring foodborne infections. Chicken gut leaving the slaughterhouses contained Campylobacter spp. This signifies the risk of crosscontamination while handling of poultry in our kitchen. As the intestinal tract of chicken, especially the cecum and colon can harbor a large number of Campylobacter spp. as commensal microflora, during processing the intestinal tract may leak or rupture and the contents are transferred to the skin of the carcass. Thus it is likely to found Campylobacter spp. in meat (poultry carcass) if contamination occurs. However, the supermarkets provide facilities for raw chicken storage and displaying in chilled or frozen condition. This is proof that efforts are being taken to control the quality and safety of the raw chicken sold in those premises. But it is often that if one part of the chicken has been contaminated, there are chances that the organism will spread to other parts as well. Thus, it is not surprising to find that even the inner parts of the chicken were also contaminated, most likely due to cross-contamination during processing.
The chilled meat samples were taken from various supermarkets where chicken meat was stored and displayed in chilled condition. Supermarkets, usually offer chicken meat under conditions that appear more hygienic than those in wet markets with better control of the storage temperatures. Even though foodstuffs in supermarkets appear more hygienic, the preparation before packing could be unhygienic and the packing facilities itself could be in poor conditions.
Chicken nuggets showed the absence of Campylobacter spp. Failure of isolating the organism could be either due to a high concentration of salt and other ingredients contained in the product or the nuggets have gone through proper handling process. Campylobacter can usually survive up to 2.0% of salt. So in this case, it was possible that the samples might have been contaminated by C. jejuni, but later was killed by the use of salt and spices. Another reason might be they had undergone minimal cooking process prior to freezing. This may have reduced the risk of occurrence of Campylobacter.
Microscopic examinations and biochemical tests were done to presumptively identify the Campylobacter spp. Hippurate hydrolysis test was done to differentiate between C. jejuni and other species. As the samples showed positive result for hippurate hydrolysis test other than raw chilled meat, it can be inferred that C. jejuni might be present in sausages and chicken gut. Other species such as C. coli, C. lari, C. fetus, C. upsaliensis might be present in raw chilled meat as these do not hydrolyze hippurate. Again it should be kept in mind that Campylobacter spp. may occasionally be misclassified when species identification is based on a single phenotypic trait such as the hydrolysis of hippurate. Molecular analysis should be performed to confirm at species level identification. In a study, it was reported that C. jejuni was found in various parts of fresh and chilled raw chicken but the absence of C. jejuni was reported for the analysis done on marinated raw chicken meat and frozen chicken-based products [7].
Consumption of insufficiently cooked frozen poultry products poses the risk of acquiring foodborne infection caused by Campylobacter spp. Supermarkets provide healthier environment than wet market to store chilled raw chicken but the packaging itself may be the source of contamination. Improper handling of poultry in the kitchen may cause crosscontamination in the kitchen as Campylobcater reside as the normal microbiota of gastrointestinal tract of poultry.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Sultana S (2017) Detection of Campylobacter spp. from Poultry and Poultry Products in Dhaka, Bangladesh. J Network Med Target Ther 1(1): dx.doi.org/10.16966/2577-1906.101
Copyright: © 2017 Sultana S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access