Abstract
Introduction: The relationship between trace elements and Oxidative Stress (OS) in Chronic Kidney Disease (CKD) patients is still not completely elucidated. The aim of this work is to determine the serum levels of OS and the trace elements in CKD patients.
Methods: A total of 91 patients were enrolled and then divided into 5 groups at the basis of CKD stages and therapy methods. Healthy volunteers were included as a control group. Serum levels of Super Oxide Dismutase (SOD), Malondialdehyde (MDA), Zinc (Zn), Copper (Cu), Calcium (Ca), Ferrum (Fe), and Magnesium (Mg) were determined, and the correlation among these data was analyzed. A portion of patients are under hemodialysis and peritoneal dialysis. Meanwhile, the association between OS and trace elements will be investigated.
Results: The MDA level in the patients’ sera was significantly higher than the controls’ (P <0.05); however, their sera level of SOD was obviously lower (P <0.05). Meanwhile, the creatinine level in CKD patients’ sera was positively correlated with the MDA level (r=0.534, P <0.01), while negatively with the expression of SOD (r=-0.427, P <0.01). A lower expression of Zn and Ca values could be seen in the cases’ sera (P <0.05). Besides that, it showed a significant negative correlation between serum levels of MDA and Ca (r=-0.282, P <0.01), MDA and Zn (r=-0.358, P <0.01), while a positive correlation between MDA and Cu (r=0.236, P=0.022). Adversely, the expression of SOD in the sera of cases was positively related to the levels of Ca (r=0.273, P <0.01) and Zn (r=0.277, P <0.01).
Conclusion: Abnormalities of OS and Zn, Ca existed in CKD patients; and elevated OS may play a role in the trace elements imbalance. Dialysis not only hemodialysis but also peritoneal dialysis seems to have no obvious effect on plasma trace elements. Correcting these disorders requires developing new ways of treatments.
Keywords
Chronic kidney disease; Oxidative stress; Trace element
Introduction
Chronic Kidney Disease (CKD) is characterized by progressive loss of kidney function, and has turned into a major global health problem. The incidence and mortality of CKD have increased over the past few decades [1]. A Chinese survey in 2012 found that the overall prevalence of CKD in adults was approximately 10.8%, placing a huge burden on health care resources [2].
Oxidative stress (OS) refers to an imbalance between the formation of Reactive Oxygen Species (ROS) and antioxidative defense mechanisms. In CKD patients, OS is an important risk factor for the development and progression of several complications. There is good evidence indicating that uremia in general is associated with enhanced oxidative stress, and treatment of uremic patients with Hemodialysis (HD) has been suggested to particularly contribute to OS and reduced antioxidant levels [3,4].
Trace elements, which are measured in microgram per gram or less, occur in minute amounts and are indispensable for our body. In some instances, they are the key components of many enzymatic pathways [5], thereby play important physiological and biochemical functions. Many studies confirmed that patients with CKD are often at risks of developing severe trace elements imbalances [6,7].
Dialysis, including HD and Peritoneal Dialysis (PD), is the most common and significant method of treatment for End-Stage Renal Disease (ESRD). Research involving the changes in trace elements in the blood has been reported in Chronic Renal Failure (CRF) patients, especially in those treated by HD [8]. The effect of trace elements on metabolism may be exacerbated in ESRD patients on dialysis because these patients are incapable of excreting excess elements properly [9]. However, the incidence of abnormal trace element status in different stages of CKD patients, including CKD3, CKD4, CKD5, especially in dialysis patients, has not been comprehensively studied.
In this study, we investigated the plasma trace element levels of Zn, Cu, Ca, Fe, Mg and OS parameters in CKD patients. We also evaluated plasma MDA and SOD levels, which are the key markers of OS. The relation between OS and those trace elements are investigated as well.
Materials and Methods
Patients
All cases were recruited from the Department of Nephrology and Blood Purification Center in the Second Affiliated Hospital of Xi’an Jiaotong University. The primary disease for all patients was chronic glomerulonephritis, and the inclusion criteria were as follows: stable blood pressure, absence of complicated infection and heart failure within the last 3 months, not pregnant or breast feeding, absence of severe cardiovascular and mental disorders. The patients were not taking any immunosuppressive agents.
A total of 91 patients were enrolled and divided into 3 groups based on the CKD stages calculated from Glomerular Filtration Rate (GFR). Using the MDRD equation to calculate the estimated GFR, patients are grouped into CKD3, CKD 4 and CKD5 which were further divided into 3 subgroups: Non-dialysis uremic group (NHD), hemodialysis group (HD) and peritoneal dialysis group (PD). The patients in the HD group had undergone regular HD treatment (4-hour treatment given 3 times a week) for more than 3 months using a dialysis solution sugar-free, Ca2+ 1.5 mmol/L, K+ 2.0 mmol/L). The dialysis membrane was polysulfone with an area of 1.4-1.8 m2, the blood flow was set to 220-250 mL/min and the dialysate flow at 500 mL/min. The PD patients had undergone regular treatment for more than 3 months using low-calcium peritoneal dialysis solution, using a standard Tenckhoff catheter, and the daily volume of replacement fluid was 8000 mL.
Twenty healthy volunteers were recruited as the control group
This study was approved by the Ethics Committee in the Second Affiliated Hospital of Xi’an Jiaotong University. Written informed consent was obtained from all patients and healthy volunteers participated in the study.
Sample preparation
Fasting hemospasia was performed in all participants. Blood samples in the HD group were collected prior to hemodialysis on the day of treatment. A routine blood test that included a complete blood count and routine clinical chemistry tests were performed. 10 mL of peripheral blood was centrifuged at 3000 rpm for 5 min and the supernatant was collected and stored at -80°C.
Enzyme-Linked Immunosorbent Assay (ELISA)
All serum samples were stored at -80°C and processed all at once. Serum levels of Cu, Ca, Fe, Zn, Mg (Xi’an microelement inspection institute), SOD and MDA (Westang, Shanghai, China) were determined by ELISA using specific detection reagents following the manufacturer’s instruction. Each assay was performed in triplicate and the experiments were repeated once.
Statistical analysis
Data were analyzed using SPSS 16.0 Statistical Analysis software (SPSS Inc., Chicago, IL, USA). Measurement data are expressed as mean ± Standard Deviation (SD). Comparison of means among groups was made by one-way analysis of variance (ANOVA), and comparison between two groups was made by Fisher’s Least Significant Difference (LSD) test. Spearman’s rank-order correlation was used for bivariate correlation analysis. A P value less than 0.05 (P <0.05) was considered statistically significant, and P <0.01 indicated a more significant difference.
Results
Patient’s characteristics
Clinical characteristics of CKD patients: A total of 91 patients were classified based on the following CKD stages: CKD3 (12 males and 10 females, mean age: 58.9 ± 19.0 years), CKD4 (10 males and 9 females, mean age: 58.3 ± 13.1 years), and CKD5 which were further divided into NHD group (8 males and 6 females, mean age: 54 ± 20.1 years), HD group (9 males and 9 females, mean age: 48.3 ± 17.5 years) and PD group (9 males and 9 females, mean age: 46.6 ± 15.0 years). Healthy volunteers were recruited as the control group (12 males and 8 females, mean age: 48.9 ± 11.4 years).
Routine blood and clinical chemistry tests were performed. Complete blood count was measured. Results were analyzed for each group and comparisons were made between groups (Table 1). Significant differences between the controls and the different stage of CKD patients were observed (Table 1). Also, there were significant differences in hemoglobin, serum creatinine, urea nitrogen, Cystatin-C, parathyroid hormone among CKD groups.
| Item |
Control (n=20) |
CKD3 (n=22) |
CKD4 (n=19) |
CKD5 (n=50) |
| |
|
|
|
ND (n=14) |
HD (n=18) |
PD (n=18) |
| Age |
48.9 ± 11.4 |
58.9 ± 19.0 |
58.3 ± 13.1 |
54.0 ± 20.1 |
48.3 ± 17.5 |
46.6 ± 15.0 |
| Gender (male/female) |
12/8 |
12/10 |
10/9 |
8/6 |
9/9 |
10/8 |
| WBC (10^12/L) |
6.17 ± 1.67 |
7.06 ± 3.21 |
5.57 ± 1.57# |
5.65 ± 1.63 |
5.64 ± 1.28 |
6.07 ± 2.10 |
| RBC (10^12/L) |
4.62 ± 0.57 |
3.46 ± 0.82* |
2.81 ± 0.65*# |
2.55 ± 0.68*# |
3.30 ± 0.72*& |
2.89 ± 0.69*# |
| PLT (10^9/L) |
235 ± 51.7 |
161 ± 58.5* |
167 ± 61.4* |
145 ± 60.7* |
157 ± 46.3* |
164 ± 94.0* |
| Hb (g/L) |
135 ± 15.5 |
104 ± 26.0* |
82.8 ± 18.1* |
77.5 ± 12.7*# |
101 ± 21.3*※& |
85.3 ± 20.0*# |
| FPG (mmol/L) |
5.10 ± 0.74 |
5.10 ± 0.98 |
4.75 ± 0.52 |
4.86 ± 2.09 |
4.28 ± 0.76$ |
5.47 ± 2.56 |
| Scr (μmol/L) |
69.6 ± 11.6 |
267.2 ± 112.3* |
556.6 ± 80.3*# |
983.4 ± 372.7*#※ |
897.3 ± 242.2*#※ |
851.6 ± 218*#※ |
| BUN (mmol/L) |
4.30 ± 0.93 |
14.5 ± 7.01* |
20.0 ± 5.94*# |
25.8 ± 9.50*#※ |
26.1 ± 7.64*#※ |
20.7 ± 8.12*# |
| Cystatine-C (mg/L) |
0.77 ± 0.16 |
3.42 ± 1.90* |
5.31 ± 1.86*# |
5.84 ± 1.94*# |
8.22 ± 4.10*#※&$ |
6.06 ± 1.09*# |
| UA (μmol/L) |
276.0 ± 89.4 |
400.7 ± 136.4* |
370.7 ± 108.1* |
412.2 ± 158.4* |
400.2 ± 83.8* |
434.2 ± 119.8* |
| PTH (pg/mL) |
38.0 ± 14.8 |
129.3 ± 131.7* |
203.8 ± 87.1* |
274.7 ± 129.2*# |
397.3 ± 349.5*#※ |
319.5 ± 136.9*#※ |
| ALT (IUI/L) |
25.8 ± 10.8 |
18.3 ± 15.3 |
17.9 ± 12.8 |
20.7 ± 9.6 |
12.8 ± 6.1 |
13.4 ± 7.1 |
| AST (IUI/L) |
19.4 ± 3.8 |
23.7 ± 17.8 |
19.8 ± 8.9 |
23.5 ± 7.2 |
14.1 ± 5.1 |
15.8 ± 6.2 |
| TP (g/L) |
71.6 ± 3.26 |
64.4 ± 9.68* |
66.9 ± 9.0 |
62.1 ± 7.82* |
65.3 ± 4.64* |
54.5 ± 5.17*#※&§ |
| ALB (g/L) |
45.3 ± 2.73 |
38.4 ± 6.43* |
38.9 ± 6.69* |
37.7 ± 5.93* |
39.5 ± 4.39* |
29.9 ± 4.76*#※&§ |
| Ca (mmol/L) |
2.01 ± 1.09 |
2.03 ± 0.34 |
1.95 ± 0.29 |
1.87 ± 0.22 |
1.92 ± 0.29 |
1.67 ± 0.23*#※§ |
| K (mmol/L) |
4.32 ± 0.44 |
4.55 ± 0.79 |
4.55 ± 0.96 |
5.07 ± 0.65* |
5.28 ± 0.76*#※ |
3.90 ± 1.16#※&§ |
| CRP (mg/L) |
6.8 ± 0.66 |
12.6 ± 0.78* |
15.7 ± 0.58* |
18.2 ± 0.46*# |
19.4 ± 0.57*# |
15.7 ± 0.61* |
| TC (mmol/L) |
4.45 ± 0.97 |
4.50 ± 1.13 |
4.40 ± 1.34 |
3.94 ± 0.48 |
3.72 ± 0.67*# |
4.14 ± 0.95 |
| TG (mmol/L) |
1.85 ± 1.39 |
1.99 ± 1.24 |
1.34 ± 0.94 |
1.28 ± 0.31# |
1.13 ± 0.44# |
1.12 ± 0.59# |
Table 1: Clinical characteristics of all CKD patients.
Values are expressed as mean ± SD.
RF: Renal Failure; NHD: Not Hemodialysis; HD: Hemodialysis; PD: Peritoneal Dialysis; Scr: Serum Creatinine; BUN: Urea Nitrogen; WBC: Leukocyte; RBC: Erythrocyte; PLT: Blood; Hb: Hemoglobin; TP: Total Protein; UA: Urico Acid; ALB: Albumin; TC: Total Cholesterol; TG: Triglyceride; PTH: Parathyroid Homone; FPG: Fasting Plasma Glucose; ALT: Glutamic-Pyruvic Transaminase; AST: Glutamic Oxalacetic Transaminase; CRP: C-Reaction Protein.
* p <0.05 vs control; #p <0.05 vs CKD3; ※p <0.05 vs CKD4; & <0.05 vs NHD; § <0.05 vs HD; $p <0.05 vs PD.
All the differences were calculated by the LSD test.
There were no significant differences in age and gender distributions between the controls and patients in different CKD stages (Table 1).
Expression profiles of serum cytokines: We evaluated the correlation of serum levels of the OS markers with creatinine levels. Serum levels of SOD (Figure 1A) and MDA (Figure 1B) were respectively significantly lower and higher in CKD patients than in controls (P<0.05), and positively correlated with advancing CKD stage (P <0.05). Serum levels of SOD were altered by dialysis treatment compared with non-dialysis patients. In contrast, serum levels of MDA were not altered by dialysis treatments.
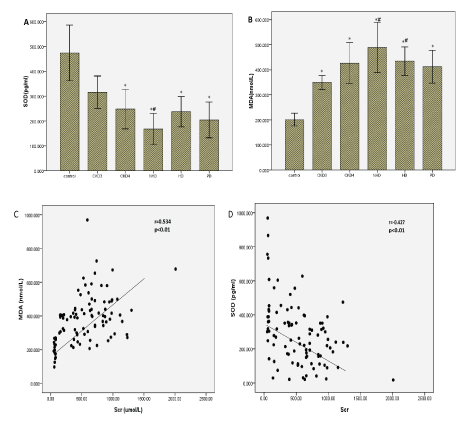
Figure 1: Serum concentrations of OS markers in CKD patients and normal control subjects.
(A) The concentration of SOD, (B) The concentration of serum MDA, The correlation between serum creatinine level and the MDA, (C) and SOD, (D) concentrations was analyzed by Spearman’s rank correlation and regression test.
The p value and r value are indicated in the graphs.
Moreover, serum creatinine levels in serum CKD patients correlated positively with serum MDA levels (r=0.534, P <0.01), and negatively with serum SOD levels (r=-0.427, P <0.01) (Figure 1C and 1D), reflecting an association between the extent of OS and the severity of renal damage (Table 2).
| Item |
Control (n=20) |
CKD3 (n=22) |
CKD4 (n=19) |
CKD5 (n=50) |
| |
|
|
|
ND (n=14) |
HD (n=18) |
PD (n=18) |
| SOD |
473.62 ± 231.08 |
315.79 ± 153.18 |
248.32 ± 173.96* |
168.12 ± 98.89*# |
237.39 ± 117.17* |
204.53 ± 124.47* |
| MDA |
201.15 ± 52.43 |
349.26 ± 66.17* |
426.28 ± 179.28* |
488.73 ± 158.34*# |
434.49 ± 109.12*# |
411.86 ± 113.5* |
Table 2: Serum concentrations of OS markers in CKD patients and normal control subjects.
SOD: Super Oxide Dismutase; MDA: Malondialdehyde
* p <0.05 vs control; #p <0.05 vs CKD3; ※p <0.05 vs CKD4; & <0.05 vs NHD; § <0.05 vs HD; $p <0.05 vs PD.
All the differences were calculated by the LSD test.
Micro trace elements: Serum levels of Zn (Figure 2A), Fe (Figure 2B), Ca (Figure 2C), Cu (Figure 2D) and Mg (Figure 2E) were analyzed by ELISA. The serum levels of Zn and Ca were significantly lower in CKD patients than controls (P <0.05). Serum levels of Zn were altered by dialysis treatment compared with no-dialysis. In contrast, serum levels of Ca were not altered by dialysis treatment. There were no significant differences in Fe, Cu, and Mg between CKD patients and controls (Table 3).
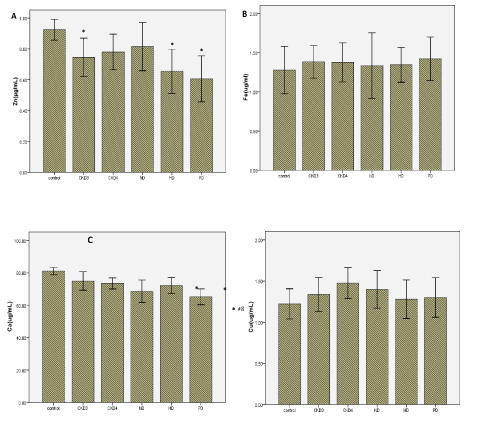
Figure 2: Serum concentrations of Micro trace elements in CKD patients and normal control subjects. (A) The concentration of Zn, (B) The concentration of Fe, (C) The concentration of Ca, (D) The concentration of Cu, (E) The concentration of Mg.
| Item |
Control (n=20) |
CKD3 (n=22) |
CKD4 (n=19) |
CKD5 (n=50) |
| |
|
|
|
ND (n=14) |
HD (n=18) |
PD (n=18) |
Zn |
0.925 ± 0.135 |
0.746 ± 0.290* |
0.781 ± 0.252 |
0.815 ± 0.247 |
0.655 ± 0.278* |
0.607 ± 0.259* |
Fe |
1.280 ± 0.602 |
1.383 ± 0.490 |
1.377 ± 0.546 |
1.334 ± 0.662 |
1.345 ± 0.430 |
1.423 ± 0.482 |
Ca |
81.06 ± 4.504 |
74.97 ± 13.30 |
73.51 ± 7.470* |
68.66 ± 11.02* |
72.15 ± 9.54* |
65.27 ± 8.420*#※ |
Mg |
19.48 ± 2.403 |
22.50 ± 4.037 |
21.73 ± 4.717 |
21.27 ± 4.666 |
22.82 ± 4.589* |
21.91 ± 4.25 |
Cu |
1.222 ± 0.368 |
1.336 ± 0.487 |
1.475 ± 0.411 |
1.398 ± 0.361 |
1.280 ± 0.454 |
1.300 ± 0.416 |
Table 3: Micro trace elements in CRF patients and normal control subjects (ug/ml).
*p <0.05 vs control; #p <0.05 vs CKD3; ※p <0.05 vs CKD4; & <0.05 vs NHD; § <0.05 vs HD; $p <0.05 vs PD.
All the differences were calculated by the LSD test.
Correlation between SOD, MDA with Micro trace elements: There was a significant and positive correlation between serum levels of SOD and Zn (Figure 3A, r=0.277, P <0.01), Ca (Figure 3B, r=0.273, P <0.01). Adversely, there was a significant and negative correlation between MDA and Ca (Figure 3C, r=-0.282, P <0.01, Zn (Figure 3D, r=-0.358, P <0.01), as well as a positive correlation between MDA and serum Cu, which did not quite reach statistical significance (Figure 3E, r=0.236, P=0.022). Other trace elements have no obvious correlation with SOD and MDA.
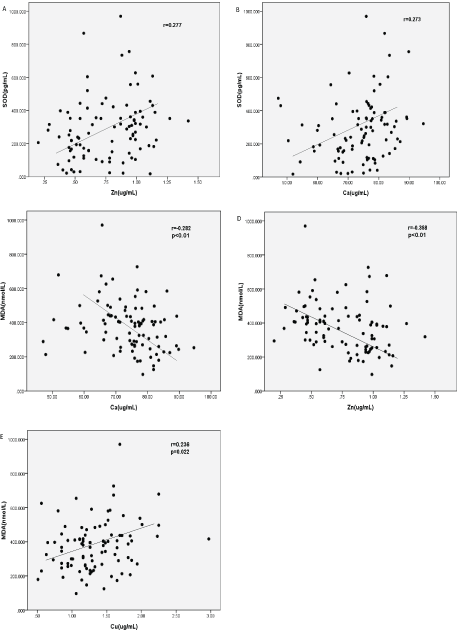
Figure 3: Correlations between OS markers with trace elements in CKD patients. Correlations were analyzed by Spearman’s rank correlation and regression tests. (A) Relationship between the concentrations of SOD with Zn, (B) Relationship between the concentrations of SOD with Ca, (C) Relationship between the concentrations of MDA with Ca, (D) Relationship between the concentrations of MDA with Zn, (E) Relationship between the concentrations of MDA with Cu.
The p value and r value were indicated in the graphs.
Discussion and Conclusion
With recent studies in pathophysiology, inflammation and dysregulated immune responses have emerged as important factors contributing to the gradual loss of renal function in CKD patients [10]. Moreover, the risk of heart diseases and cancers increases markedly in CKD patients [11,12]. Also, the CKD patients are at the risk of developing severe trace elements imbalances [13,14]. Trace elements are indispensable and often combined with proteins, coenzyme, hormones and vitamins. In ESRD patients, many different factors affect the serum concentrations of trace elements, such as increased oral intake, failure of renal excretion, loss of appetite, metabolic alterations and so on. During dialysis, some trace elements can accumulate because of dialysis fluid impurities, and others may move from blood to the dialysate, leading to a deficiency [15].
In our study, plasma Fe, Cu and Mg trace element levels in CKD patients were compared with the control group and between each other. We found that plasma Fe levels had statistically no differences between patients and the control group. This may be attributed to the fact that we often supplement Fe in CKD patients because many patients are anemic, especially in CKD5. Again, there were no differences in plasma Cu and Mg. We analyzed that the control and patients had similar regional changes and dietary habits directed at soil and water contamination. Adversely, Serum Zn and Ca values were significantly lower in our CKD patients than the controls, and the tendency became more obvious as the disease progressed, especially in CKD5 patients. Zn is an essential component of various proteins and biomembranes [16]. Recent studies suggest that the increased urinary zinc excretion and decreased intestinal Zn absorption may be the primary cause of hypozincemia in patients. Also dietary restrictions and anorexia lead to an insufficient micronutrient intake. Shrimpton R, et al. [17] argued that zinc deficiency is a leading cause of diseases in developing countries and is associated with immune deficiencies by impaired cell proliferation, abnormal T-cell function, and cytokine expression, all of which might contribute to the high risk of infection observed in uremia patients. Meanwhile we found lower levels of Zn in HD and PD lower than other CKD patients. We think it is because these patients have more advanced disease requiring renal replacement therapies, which consumes more Zn. Ca is one of the most abundant mineral elements in human body, and the normal function of tissues and organs depends on its existence. The person can become sensitive, emotional instability and attention deficit if they lack sufficient Ca levels. In CKD patients, the kidney damage leads to phosphorus excretion. On the other hand, a diet with an excessive intake of phosphorus combined with Ca, leads to depositions and a loss in Ca levels, creating an insufficient production of 1.25(OH)2vitD3, which reduce Ca absorption. We found that the decrease in serum Ca is more significant in PD patients, which are more likely to use a low Ca dialysis fluid. These findings are consistent with several other investigators [18]. Miura Y, et al. [19] compared serum Mg and Cu levels between HD patients and control groups and found no statistically significant differences. The results of the Miura study also found that Zn is lower in HD patients. But Krachler M, et al. [20] found elemental composition changes in plasma and observed a decrease in Mg levels over a 6-month period in uremia patients. Regulating trace element levels is essential to prevent complications in HD patients. Differing results have been reported with respect to plasma levels of trace elements in HD patients. Tonelli, et al. found low plasma Zn and high plasma Cu levels in HD patients compared with controls. The distribution of trace elements can be affected by insufficient dietary intake, anorexia, degrees of gastrointestinal absorption, and drug treatment, which can lead to clinical abnormalities in uremic patients [21,22]. The uremic state, medication, the dialysis process, dietary intake, and the quality of water used for dialysis may contribute to such clinical abnormalities in trace elements [23]. Although recent improvements in technology in performing dialysis can prevent some serious complications by changes in trace elements; however, problems still remained [24]. In our study, although dialysis treatment improved the survival of CKD patients, it had a limited effect on trace elements. Dialysis augments reduced renal function by removing metabolic waste products and maintaining the electrolyte balance and acidbase equilibrium. However, due to the possible risks present in HD, such as blood loss during treatment, dietary restrictions, anorexia, aluminum intoxication [25] and dialyzer biocompatibility [26], there is no minimal change in the balance of trace elements when compared to non-dialysis patients. Although PD has advantages over HD in these respects [27], problems such as increased protein loss, excessive glucose intake and triglyceride abnormalities make it ineffective in restoring immune balance. Further investigation is required to identify new therapeutic agents or strategies to improve the patient’s microenvironment.
OS refers to an imbalance in the body’s oxidation and antioxidation, resulting in neutrophil inflammatory infiltrates, increases in proteases secretion, and produces a large number of oxide intermediates. Several studies have confirmed that the occurrence of complications in CKD such as accompany hypertension [26] and diseases affecting the heart and nervous system. OS is one of the important risk factors affecting prognosis of CKD [28]. SOD and MDA can be used to evaluate the body’s resistance to oxidation and peroxidation levels. In our study, the serum levels of MDA were significantly higher and SOD significantly lower in CKD patients than in controls. Meanwhile we found that serum levels of MDA and SOD were closely related to serum creatinine levels. The results reiterate that the severity of CKD correlates with the degree of OS. However, as the renal function deteriorates, OS response might impair renal function and the extent of renal damage could conceivably increase during progression of CKD. So we believe that the OS reaction, caused by a metabolic disorder and apoptosis, may be involved in the developmental process of renal fibrosis. But serum levels of SOD were altered by dialysis treatment compared with no dialysis; therefore, replacement therapy may improve the imbalance in the body’s oxidation and antioxidation states in CKD patients.
We also compared trace element levels and oxidant–antioxidant parameters and found that in patient groups, there was a significant positive correlation between serum levels of SOD with Zn (Figure 3A, r=0.277, P <0.01), Ca (Figure 3B, r=0.273, P <0.01). Adversely, there was a significant negative correlation between serum levels of MDA with Ca (Figure 3C, r=-0.282, P <0.01, Zn (Figure 3D, r=-0.358, P <0.01) as well as a positive correlation between MDA and serum Cu, but the relationship did not quite reach statistical significance. According to the above description, OS is one of the important risk factors affecting prognosis of CKD, so we have reasons to regard that Zn and Cu are closely influenced by OS. Cu is a transition element and a cause of radical ROS formation through the Fenton reaction. These ROS radicals cause lipid peroxidation, an end product of MDA. Consistent with our study, Rükgauer M, et al. [29], analyzed trace elements and MDA concentrations in healthy persons and when comparing Cu levels and MDA concentrations, there were no correlations between these two parameters. Other trace elements (Mg and Fe) have no obvious correlation with SOD and MDA.
According to our results, elevate OS may play a role in the imbalance in trace elements in CKD. Dialysis itself seems to have no obvious effect on serum levels of trace element concentrations when compared to non-dialysis CKD5 patients. Therefore, decreasing sources of OS and at the same time increasing oxidative capacity can be beneficial to these patients. These findings show that whereas prolonged replacement treatment leads to improved OS damage, trace element concentrations are not affected. Both deficiency and the excess in trace elements are potentially harmful yet amenable to therapy The hypothesis that trace elements influence the risk of adverse clinical outcomes is worthy of investigation. It is critical that micronutrient interventions are not only affected, but also a target for those with the greatest need.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Human Research Committee for Research Involving Human subjects in the Second Affiliated Hospital of Xi’an Jiaotong University and the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
The study was approved by the local ethical committee and all patients gave informed consent.
Conflict of Interests
The authors declare no financial or commercial conflict of interests.
Acknowledgements
This work was supported by the Science and Technology Program of Xi’an [No. 2019114613YX001SF037 (2)] and Science and Technology Project of Shaanxi Province (No. 2016SF-329). We thank the Department of Nephrology in the Second Affiliated Hospital of Xi’an Jiaotong University for providing the peripheral blood samples. We express our gratitude to all the study participants.




