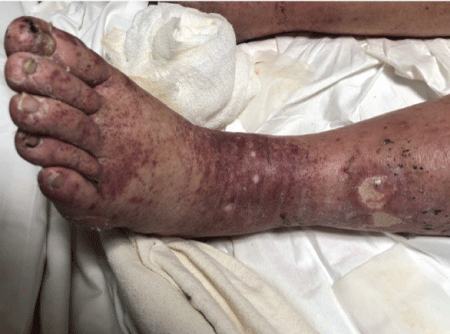
Figure 1: Left leg of the patient showing the purpuric rash.


Deepti Avasthi1* Deepak Malhotra1 Salil Avasthi2 Riddhish Sheth3 Maria Alfonso-Jaume1
1Department of Internal Medicine, Nephrology sub division, University of Toledo Medical Center, Ohio, USA*Corresponding author: Deepti Avasthi, Department of Internal Medicine, Nephrology sub division, University of Toledo Medical Center, Ohio, USA, E-mail: Dipti312@gmail.com
Henoch-Schonlein-Purpura with Nephritis (HSPN) is characterized by IgA Nephritis (IGAN), Non-Thrombocytopenic Purpura, Intestinal inflammation and Arthritis. It is well established that HSPN most commonly occurs after a bacterial or viral infection in respiratory tract. However, there is still a dearth of knowledge about the etiopathogenesis of this disease and its relationship with Autoimmune Diseases. Our manuscript describes a case of HSPN, IgA Monoclonal Gammopathy of Unknown Significance (MGUS) in a patient with long standing history of Rheumatoid Arthritis (RA). This rare presentation makes for a stimulating topic to discuss the underlying pathogenesis of these three diseases. It also supports the hypothesis that molecular changes in RA can lead to MGUS and IGAN. We have performed a thorough literature search and written a detailed description of pathological relationship between these three diseases.
74 y old male presented with palpable purpura on his lower extremities for 3 weeks. The rash was severe and rapidly progressed to involve the upper extremities and chest. There was an associated diffuse arthralgia, mild to moderate intermittent abdominal pain and shortness of breath. His symptoms were not associated with fever, chills or cough. His past medical history was significant for Rheumatoid Arthritis and Psoriasis for 20 years and medication history included use of warfarin and furosemide. The clinical examination was significant for mild dyspnea at rest. There was diffuse palpable purpura with intermittent skin ulceration especially on the foot and distal leg (Figure 1). There was also joint tenderness and stiffness with mild joint swelling involving knees, elbows, wrist and small joints of hands and feet. Rest of his examination was unremarkable.

Figure 1: Left leg of the patient showing the purpuric rash.
A CT chest was done to evaluate his symptoms of dyspnea and was consistent with bilateral pleural effusion, lymphadenopathy and patchy pulmonary infiltrates. Pleural Fluid Analysis was done which showed a total protein level of 1.8, LDH level 90, pH 7, glucose level of less than 10 mg/dl, RBC count of 27526 and WBC count of 88. The pleural fluid culture was negative for an infection. These findings in addition to an elevated Rheumatoid factor of 29 and Anticyclic citrullinated peptide (anti CCP) of 165 were diagnostic for Rheumatoid Arthritis and Rheumatoid pleural effusion.
Other significant findings on laboratory investigations were presence of anemia (Hb 7.8 mg/dl) and an elevated BUN and Creatinine (BUN 38, Creatinine 1.88). These findings appeared acute in comparison to normal baseline hemoglobin and a normal renal function 1 year ago. The urine microscopy was positive for presence of Red Cell Casts and Urine Protein: Creatinine ratio of 0.6. There was a low complement C3 level of 44. In addition, Immunofluorescence and Serum protein electrophoresis were positive for an elevated serum IgA level of 1235 with IgA Lambda Monoclonal Band. Serological panel for ANA, ANCA, Cryoglobulins and Hepatitis panel was negative. Both blood and urine cultures were negative for any evidence of infection.
An upper GI endoscopy was done to evaluate a low hemoglobin and revealed presence of duodenitis but no source of active bleeding. For further evaluation of renal disease, we performed a bone marrow, skin and renal biopsy. The bone marrow biopsy showed plasmacytosis of 5% and ruled out a multiple myeloma. It was diagnostic for IgA Lambda MGUS. The Skin biopsy was positive for Leucocytoclastic Vasculitis and kidney biopsy had findings of Focal Segmental Mesangial Proliferative Glomerulonephritis. Further Immunofluorescence studies on renal tissue showed strong staining for Monoclonal IgA Lambda light chains and C3 deposits. There was an absence of prominent sub endothelial deposits, Cryoglobinemic Glomerulonephritis or Light Chain Deposition Disease. These findings were consistent with IgA glomerulonephritis.
Figures 2-6 are the snap shots taken from the pathology slides of the patient`s renal sample seen under light microscope and immunofluorescence.
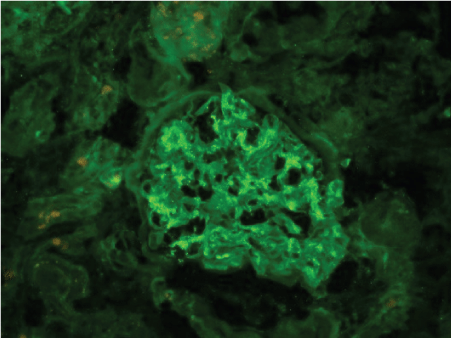
Figure 2: Immunofluorescence showing IgA deposition.
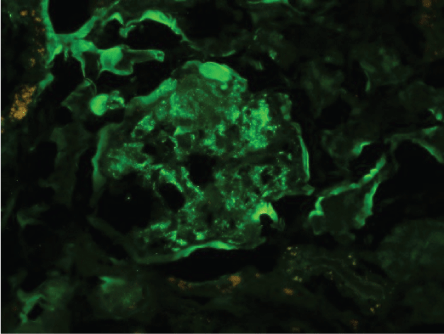
Figure 3: Immunofluorescence showing C3 deposits.
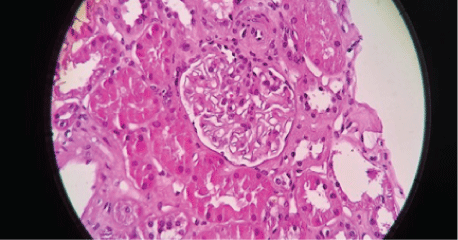
Figure 4: Segmental Mesangial Proliferation.
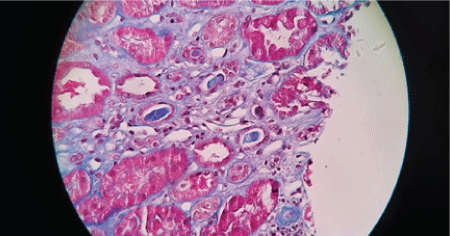
Figure 5: Red Cell Cast.
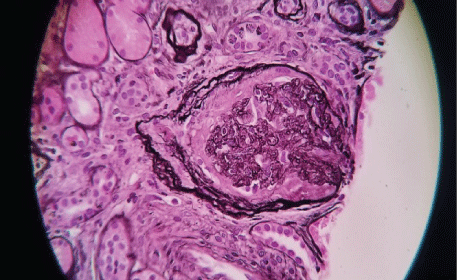
Figure 6: Crescent formation.
On the basis of clinical presentation, lab investigation and pathology findings, patient was diagnosed as having RA, IgA Lambda-MGUS and HSPN. The patient was treated with high dose prednisone 60 mg oral daily for 3 weeks. He had rapid renal recovery with return of serum creatinine to 1.3 and near resolution of his rash. On his outpatient office follow up after 3 months of his hospital discharge, he continued to have a creatinine of 1.2. He is currently off prednisone and does not wish to receive any further treatment for Rheumatoid Arthritis or his renal disease.
HSPN is a clinical diagnosis with a tetrad of IgAN, Leucocytoclastic Vasculitis of skin, inflammation of GI tract and Arthritis. The renal pathology in HSPN/IgAN is characterized by IgA deposition in mesangium with mesangial proliferation. Our case has all these features.
The research for IgA molecules first started in 1976 when Clancy and Bieenstock proved activity of IgA against viruses and bacteria [1,2]. Then in 1986 the structure of IgA was studied further and IgA was classified in 2 separate systems [3,4]:
The half-life of IgA is 6 days and the circulating IgA is cleared from blood via interaction with Asialoglycoprotein and CD89 receptor in liver and spleen [5]. The IgA molecules are large molecular weight molecules and hence are not filtered in the glomerulus.
This breakthrough finding led to a hypothesis that in an event of increase IgA levels in blood either from increased production from plasma cell dyscrasias or a decreased clearance secondary to a liver disease, the excess IgA molecules would precipitate in renal mesangium and cause HSPN/IgAN. The hypothesis gained a lot of traction when multiple case reports were published stressing association of MGUS, liver diseases and IgAN [6-14]. However, the theory was disregarded in 2000-2001 when the IgA deposits in all these cases were found to be structurally different than the normal circulating IgA molecules in a healthy person. The abnormal IgA molecules showed one of the following changes, an abnormal glycosylation of the immunoglobulin, galactose deficiency, changes in the V region, antigen binding sites or presence of an electrostatic charge [9,14,15]. This observation was reproduced and confirmed in animal models and it was derived that the abnormal IgA molecule had properties to evade the normal clearance mechanism in the liver, form IgA aggregates, activate alternative complement pathway and hence precipitate in renal and skin tissue [5-8].
The search for a complete understanding of etiopathogenesis of IgAN has since provided many strong theories about the role of certain pathogens and inflammatory disorders in initiation of this process. In the period from 1985-1987, studies in Autoimmune disorders provided new insight in the pathogenesis of the RA and its impact on the Immunoglobulins. RA was found to have a unique property to induce abnormal glycosylation of the immunoglobulins, mainly IgG and IgA in synovium. This fact is supported by identification of abnormal immunoglobins in patients with RA. Currently these abnormal immunoglobins are measured and are being studied for their prognostic value in treatment of RA [16-19]. The description of a presence of HSPN in patient with RA in various case reports [20,21] also supports the clinical relevance of this finding.
Another important impact of RA on production of immunoglobins is increased production of both polyclonal and monoclonal antibodies due to a constant antigenic stimulation of the epithelial mucosa lining the synovium, intestine and respiratory tract [22-30]. The association of Plasma Cell Dyscrasias and RA is very well established in both animal and human models [27-30].
Our patient had a very rare manifestations in the form of RA, IgA lambda MGUS, and monoclonal IgA induced HSPN. We believe that his disease process started as RA and was complicated by development of IgA Lambda MGUS and IgA Lambda vasculitis and Glomerulonephritis. These very different clinical entities have a pathological relationship with each other. Our case report raises the hypothesis of RA as a cause of IgA nephropathy. Further studies are needed to study if treating the underlying Rheumatoid Arthritis will successfully treat and prevent relapse of HSPN in these patients.
There are no conflicts of interest and the authors did not receive any funding for this manuscript.
Download Provisional PDF Here
Article Type: CASE REPORT
Citation: Avasthi D, Malhotra D, Avasthi S, Sheth R, Alfonso-Jaume M (2018) Rheumatoid Arthritis and IgA nephropathy: An Association or Coincidence? Int J Nephrol Kidney Fail 4(4): dx.doi.org/10.16966/2380-5498.164
Copyright: © 2018 Avasthi D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access