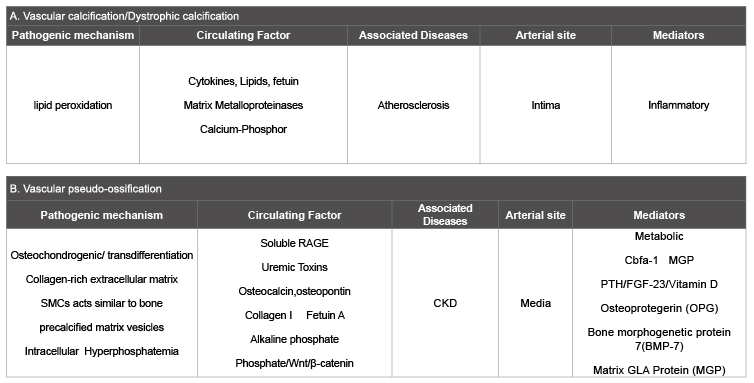
Table 1: Summary of the main characteristics of vascular calcification and vascular ossification

Farid Nakhoul Inbal Dahan Evgeny Farber Izhar Ben Shlomo*
Baruch-Padeh Poriya Medical Center, Faculty of Medicine, Bar-Ilan University, Lower Galilee 15208, Israel*Corresponding author: Izhar Ben Shlomo, MD, Baruch-Padeh Poriya Medical Center, Faculty of Medicine, Bar-Ilan University, Lower Galilee 15208, Israel, E-mail: izhar.benshlomo@gmail.com
The current paper is a Perspective & New Opinion article that offers the term “Vascular pseudo-ossification” as a new term for “vascular calcification” phenomenon in chronic kidney disease (CKD). Vascular calcification is a well-documented complication of CKD. Patients with CKD develop extensive medial calcification, which causes increased arterial stiffness that represents an independent predictor of increased morbidity and mortality due to cardiovascular events. Vascular calcification (VC), in which vascular smooth muscle cells (VSMCs) undergo a phenotypic transformation into osteoblast-like cells, is one of the emergent risk factors for the accelerated atherosclerosis process characteristic of chronic kidney disease (CKD).
Until recently, the accepted concept was that vascular calcification in CKD is a passive process of dystrophic calcification that serves as the end stage of degenerative vascular disease. However, the recent accumulation of data indicates that it is an actively regulated process which bears many similarities to bone formation. The vascular smooth muscle cells can undergo trans-differentiation into osteoblast-like cells by losing characteristic smooth muscle markers subsequently with up-regulation of osteoblastic and bone specific markers and then become calcified similarly to bone formation.
Considering the differences between dystrophic calcification and the process of vascular calcification in CKD, concurrently with its close resemblance to ossification, we suggest that the pathophysiological process that culminates in the deposition of calcium in arterial intima should rightfully be designated “Pseudo-ossification” rather than “calcification”.
Chronic kidney disease; Vascular calcification; Bone formation; Pseudo-ossification
CKD: Chronic Kidney Disease; ESRD: End-Stage Renal Disease; GFR: Glomerular Filtration Rate; VSMC: Vascular Smooth Muscle Cells; Bone Morphogenic Protein-2
Chronic kidney disease (CKD) is a progressive loss of renal function that can progress to End-Stage Renal Disease (ESRD) necessitating dialysis treatment. The disease manifestation is classified and defined as reduced glomerular filtration rate (GFR), proteinuria, and structural kidney disease. Vascular calcification (VC) is the deposition of calcium phosphate crystals in cardiovascular tissues [1]. In the past, VC was considered a passive and degenerative process [2]. However, recent evidence led to its recognition as a pathobiological process sharing features with embryonic bone formation, which promoted an increased interest in the study of VC and its clinical implications
VC is a well-documented complication of CKD. Patients with CKD develop extensive medial calcification, in which vascular smooth muscle cells undergo a phenotypic transformation into osteoblast-like cells. This transformation may play a major role in the pathogenesis of VC in the contest of passive calcium and phosphate deposition. Moreover, accelerated atherosclerosis, causes increased arterial stiffness that represents an independent predictor of increased morbidity and mortality due to cardiovascular events. There is some evidence that diabetes mellitus can contribute to VC in diabetic nephropathy patients similar to advanced age and atherosclerosis [1-4]. However, the precise mechanism behind it is unclear and needs to be determined.
The high frequency of cardiovascular disease in CKD has been associated with VC as a predictor of subsequent vascular mortality. VC in CKD is associated with oxidative stress, uremia and hyperphosphatemia leading to the formation of osteoblast-like cells in the vessel walls. Furthermore, the recruitment of progenitor cells and the expression of transcription factors essential for their osteoblastic differentiation, such as Runx2 and Msx2, promote VC in kidney disease. Elevated serum Bone morphogenic protein-2 (BMP-2) levels in patients with CKD with high phosphate also influence the osteogenic differentiation of VSMCs. Dalfino et al. [5] showed that oxidative stress promotes the differentiation of VSMCs by stimulating the release of BMP-2 by endothelial cells. Despite significant advances in our understanding of the process of VC in patients with kidney disease, the factors involved and the exact mechanism remain unclear.
Vascular disease is the most important cause of mortality and morbidity among patients with CKD, while VC is common and is nearly ubiquitous in these patients and progress rapidly. VC is an active process, involving multiple risk factors such as diabetes mellitus, high plasma phosphate and uremia, which influence the cardiovascular function and arterial stiffness. Hyperphosphatemia is the main key driver in this process [6]. As illustrated in Figure 1, the vascular smooth muscle cells undergo transdifferentiation, with loss of the characteristic smooth muscle markers such as α-smooth muscle actin [7] and develop osteoblastic features such as expression of alkaline phosphatase, osteocalcin, and osteopontin [8,9]. The recruitment of progenitor cells and the expression of transcription factors express bone specific markers, that are essential for their osteoblast differentiation, such as Runx2 and Msx2, promote vascular calcification [10,11].
Bone derived factors and the pathway that regulate their expression have recently been considered as the nucleus for cardiovascular disease associated with CKD. The Wnt/β-catenin signaling pathway is critical for the osteogenic commitment of mesenchymal cells and has been shown to be activated during VC. Rong et al. [11] in their elegant study examined the link between BMP-2, VC, and the activity of the Wnt/β- catenin signaling pathway in CKD, and showed that high phosphate up regulate β–catenin in parallel with the differentiation of VSMC apoptosis. Moreover, their results provide a possible mechanism by which alterations in Wnt/β-catenin signaling play a role in the pathogenesis of CKD by affecting the osteogenic differentiation of vascular smooth muscle cells [11].
BMP-2, another key player in bone formation, which initiates osteoblastic differentiation of mesenchymal precursor cells to induce mineral crystal precipitation into collagen fibrils, was found to accelerate arterial intimal calcification [12,13]. BMP-2 has also been involved in the mobilization of mesenchymal cells and the formation of ectopic bone [14]. Goettsch et al. [15] showed that miR-125b, an inhibitory micro-RNA which is known to regulate osteoblast differentiation, plays a role in the in vitro early phase of osteogenic trans differentiation of human coronary artery smooth muscle cells. On the bone resorption side, it was found that osteoprotegerin which acts as a soluble decoy to the activation of the receptor for RANKL, is also functional in vascular calcification [16,17], further consolidating the similarities between true bone and vascular calcification. Bone derived factors and the pathways that regulate their expression have recently been placed at the center of cardiovascular disease associated with CKD.
The uniqueness of this process in vascular walls is becoming clear when one considers dystrophic calcification. Eidelman et al. [18] concluded that dystrophic calcification in soft tissues differs significantly from bone by virtue of several characteristics. These were the presence of much less organic material than bone, low collagen content, presence of Mg and Na, apatite that is much more crystallized than bone, the mineral density of the deposits and their stiffness are higher than bone. Talmon & Wisecarver [19] documented hepatocellular post ischemic calcification as being mainly intracellular, as opposed to extracellular bone deposition. Likewise, Grases et al. [20] could not detect any role for osteopontin, a significant component of ossification processes, in the experimentally induced dystrophic calcification following subcutaneous injection with KMnO4 in rats. Differences from bone formation were demonstrated in several other forms of dystrophic calcification [21-24]. Table 1 summarized the main characteristics of VC and vascular ossification.
In the light of all the above differences between dystrophic calcification and the process of vascular calcification in CKD, and the close resemblance of the latter to ossification, we suggest that the pathophysiological process that culminates in the deposition of calcium in arterial intima should rightfully be designated “pseudo-ossification” rather than calcification.
The authors declare that they have no competing interests.
All authors discussed the concept, collected literature and together written the manuscript.

Table 1: Summary of the main characteristics of vascular calcification and vascular ossification
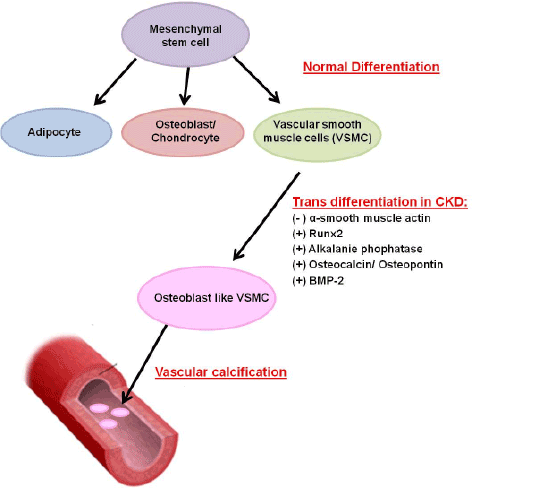
Figure 1: Vascular calcification in CKD as an actively regulated process. Normally, mesenchymal stem cells can differentiate into adipocytes, osteoblasts, chondrocytes, and vascular smooth muscle cells (VSMC). In the setting of chronic kidney disease (CKD) the VSMC can undergo trans-differentiation into osteoblast-like VSMC and express alkaline phosphatase, osteocalcin, osteopontin, Runx2 and Bone Morphogenic Protein-2 (BMP-2). These osteoblast-like VSMC then become calcified in a process similar to bone formation.
* This perspective paper is dedicated to the memory of Green Jacob M.D Rambam Medical Center, who was pioneer in this area.
Download Provisional PDF Here
Article Type: Short Communication
Citation: Ben-Shlomo I, Farber E, Dahan I, Nakhoul F (2015) “Vascular Pseudo-Ossification” rather than “Vascular Calcification” – A New Term for an Old Phenomenon in Chronic Kidney Disease: Perspective and New Opinion. Int J Nephrol Kidney Failure 1(3): http://dx.doi.org/10.16966/2380-5498.117
Copyright: © 2015 Nakhoul F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access