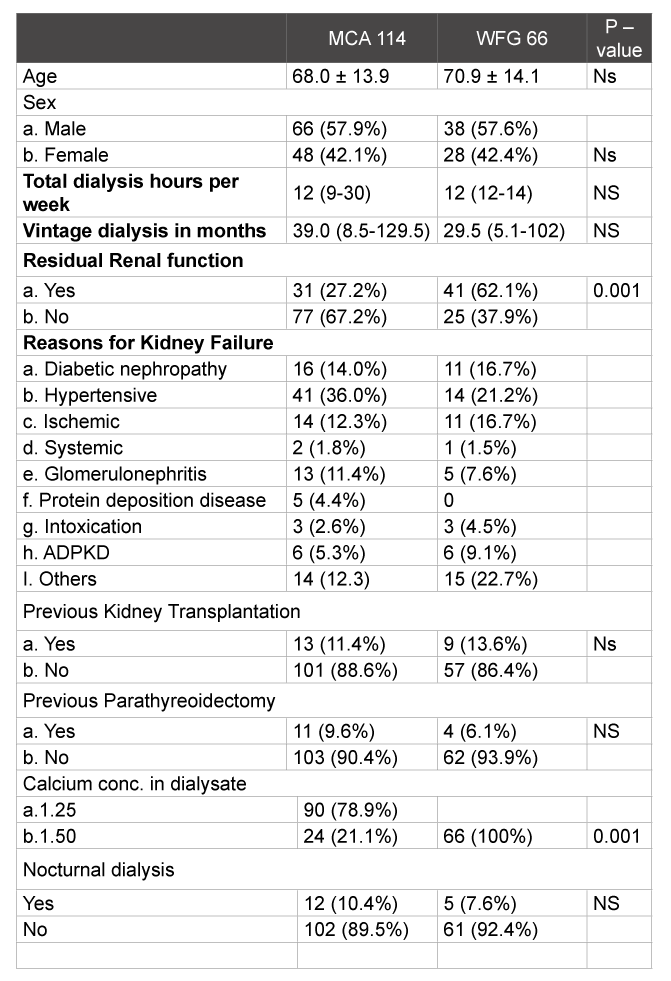
Table 1: Baseline characteristics expressed in mean with standard deviation, interquartile range (10-90 percentile) and percentages. P-value <0.05 is significant. NS= not significant.

Cost Effective Management of CKD-MBD: An Observational Study
Borefore P Jallah1*, Maarten HM Raasveld2, Bastiaan van Dam1,
1Alkmaar Medical Center, Amsterdam, The Netherlands*Corresponding author: Borefore P Jallah, Alkmaar Medical Center, Van Hogendorpstraat 159-1, Amsterdam, The Netherlands, Tel: 0654203923; E-mail: Papmeo10@hotmail.com
Chronic Kidney Disease and Mineral Bone Disorder (CKD-MBD) is often present in dialysis patients and is associated with increased hospitalization, cardiovascular morbidity and mortality. It is manifested by abnormalities in serum calcium, phosphate, parathyroid hormone (PTH) or vitamin D metabolism. Multiple interventions that often interact are possible to change biochemical parameters. The choice of treatment can greatly affect both biochemical outcomes and possibly also cost.
The aim of this study was to compare management of CKD-MBD between two centres and test whether this comparison can lead to changes in management strategy.
A prospective observational study of prevalent hemodialysis patients treated in two general hospitals in the Netherlands between September 2011 and October 2012. Treatment strategies in the management of CKD-MBD, biochemical parameters and cost effectiveness were compared. After the comparison, measures were taken in one hospital to improve cost effectiveness without compromising biochemical outcomes, increasing the total calcium load, or depleting patients from vitamin D.
In September 2011, 93 patients in the MCA and 82 patients in the WFG underwent intermittent hemodialysis. In both hospitals, CKB-MBD was managed according to KDIGO guidelines. Albumin-corrected calcium and phosphate levels were similar, while PTH was higher in the MCA at both time points. Average daily elemental calcium supplementation per patient did not differ significantly between the hospitals, though it was slightly higher in the WFG. On the contrary, within the group of patients who were prescribed calcium-based phosphate binders, the mean dose of calcium was significantly higher in the WFG (1.7 g/pt/day vs 0.7 g/pt/day in the MCA in September 2012). The total costs of treatment of CKB-MBD in euro per patient per day in the MCA and WFG differed significantly at baseline (11 vs 4 euro), but not one year thereafter (4.50 vs 5.00 euro).
In conclusion, considerable differences in CKD-MBD management can exist within KDIGO guidelines. In the absence of randomized trials, comparison of management strategies between centres can help to improve care. Cost effective management of CKB-MBD is possible without increasing calcium load or depleting patients from vitamin D.
CKD-MBD management; KDIGO guidelines; Cost effectiveness; Phosphate binder; Calcium load; Dialysis
Chronic Kidney Disease and Mineral Bone Disorder (CKD-MBD) is often present in dialysis patients and is associated with increased hospitalization, cardiovascular morbidity and mortality [1,2], It is manifested by abnormalities in serum calcium, phosphate, parathyroid hormone (PTH) or vitamin D metabolism [3-5].
Multiple interventions that often interact are possible to change biochemical parameters. For the management of CKD-MBD KDIGO guidelines (Kidney Disease: Improving Global Outcome) were first published in 2002 and revisited in 2009. Most of the commendations in these guidelines are based on observational studies and leave an opportunity for significant differences in CKD-MBD management. The choice of treatment can greatly affect both biochemical outcomes and possibly also cost. As healthcare expenses are rapidly increasing, it is important to develop cost-effective strategies for CKD-MBD management while maintaining or improving the quality of provided healthcare.
The management of CKD-MBD in two dialysis centers was compared, after which changes in one center were implemented. Effects of changes in prescription on biochemical outcome as well as cost were evaluated thereafter.
A prospective observational study of prevalent hemodialysis patients treated in two general hospitals in the Netherlands between September 2011 and October 2012, the Westfriesgasthuis (WFG) and the Medical Center Alkmaar (MCA).
The patient’s baseline characteristics that were extracted from the medical database of both hospitals were age, gender, total dialysis hours per week, vintage dialysis (including time on peritoneal dialysis), dialysate calcium concentration (at the start and at the end of the study period), residual renal function, reason for kidney failure and co-morbidities. It was also recorded if a patient had undergone parathyroidectomy or kidney transplantation in the past or during the observation period. Patients on dialysis for less than a month were excluded.
In the WFG, all patients were prescribed native vitamin D (cholecalciferol 400 IE/daily) without assessment of vitamin D levels. Alphacalcidol (0,25 µg daily) combined with calcium carbonate was only prescribed if secondary hyperparathyroidism was present. Hyperphosphatemia was treated preferably with calcium carbonate. Sevelamer and/or lanthanum were added when albumin corrected calcium rose above 2,40 mmol/l. Cinacalcet was only used for the treatment of patients with both secondary hyperparathyroidism and hypercalcemia and a contraindication for parathyroidectomy. Calcium-concentration in the dialysate was 1,5 mmol/L without variation between patients. CKD-MBD management was not changed between September 2011 and October 2012.
By September 2011, all patients in the MCA were prescribed native vitamin D in a dose of 400 IE per day, regardless their serum vitamin D level. Vitamin D levels were assessed twice yearly and a supplementary dose of 50.000 IU-100.000 IU per month was prescribed in those patients that were considered vitamin D deficient (<50 nmol/L), after which the same dose of colecalciferol was maintained. A low dose of alphacalcidol (0.25 µg/day) was prescribed in most patients, regardless of their PTH. In patients with high PTH levels, increasing doses of alphacalcidol or paricalcitol were preferably used to control PTH-levels. Calcium in the dialysate was preferably 1,25 mmol/L and was increased to 1,5 mmol/L in case of hypocalciemia. Hyperphosphatemia was managed preferably with calcium free phosphate binders, primarily sevelamer or lanthanum.
During the study observation period, monthly doses of cholecalciferol were increased or decreased to achieve target levels between 50-100 nmol/L. In patients with increasing PTH levels, an increasing dose of calcium, rather than an increasing dose of alphacalcidol was preferably used, either by increasing calcium in the dialysate to 1.5 mmol/L or changing calcium-free to calcium-containing phosphate binders. In patients with secondary hyperparathyroidism combined with otherwise untreatable hypercalcemia either the conversion of alphacalcidol to paricalcitol or the addition of cinacalcet was considered.
Other changes introduced in the Medical Center Alkmaar during the observation period were the replacement of calcium carbonate with calcium acetate combined with magnesium. Paricalcitol was converted to alphacalcidol and cinacalcet was reduced in patients when PTH levels as well as serum corrected calcium were within KDIGO targets. These changes were implemented gradually for all the patients present and those initiating dialysis during this period.
The following medications prescribed in the management of CKDMBD were recorded at given periods (September 2011, December 2011, March 2012 and September 2012): calcium free phosphate binders (Sevelamer, Lanthanum and magnesiumoxide/hydroxide), calcium based phosphate binders (calciumacetate, calcium carbonate), native vitamin D (Cholecalciferol), vitamin D analog/sterol (Alfacalcidol) and paricalcitriol (Zemplar) and calcimimetics (Cinacalcet).
Biochemical parameters (calcium, albumin, phosphate and PTH) were also recorded one month after changes in the medications were implemented (October 2011, January 2012, April 2012 and October 2012). Vitamin D was measured in April and October of 2012.
The cost of MBD medication per patient per day was calculated using the average daily dosages of medication and the average patient population present. The costs per gram used in the calculation of cost were Euro 1,35 for Sevelamer, Euro 2,46 for lanthanum, Euro 0,07 for magnesiumoxide, Euro 0,9 for each gram of calcium in calciumacetate (either in Phoslo or in Osvaren), Euro 0,5 for each gram of calcium in calciumcarbonate, Euro 6,7 for 30 mg of cinacalcet, Euro 0,16 for 0.25 µg of alphacalcidol and Euro 26,2 for 5 µg of paricalcitol.
Assays for measurement of calcium, phosphorus and PTH were similar in both hospitals, but albumin assays differed between the hospitals. In the WGF, albumin was measured using the BCG method and in the MCA using the BCP method. Intact PTH (1.3-6.8 pmol/l) and phosphate (0.7- 1.4 mmol/l) were measured using UniCEL DXI (600). Vitamin D was measured in the MCA using IDS-ISYS analyzer with a reference range between 50-150 nmol/l.
Statistical analysis was performed using SPSS 20 (SPSS Inc., Chicago, IL, USA). Values are expressed as means plus standard deviation, median plus inter-quartile range (10-90 percentile) or as percentages. Non parametric test were used in the calculation of the p-values as significant variations in values were present and the patient population varied over the study period. A p-value < 0,05 was considered statistically significant.
Between September 2011 and October 2012, a total of 114 patients in the MCA and 66 patients in the WFG underwent chronic intermittent hemodialysis at one time point or another. In both general hospitals, management of CKB-MBD was in accordance with KDIGO guidelines. Baseline characteristics (Table 1) were generally comparable except for the number of patients with residual renal function. There were significantly more patients in the WFG with residual renal function (defined as >200 ml diuresis per 24 hours with/without diuretics).
Median duration of dialysis per week was twelve hours. Thirteen patients in the MCA had previous kidney transplantation, 15 patients (13.2%) were transplanted during the study period. Nine patients in the WFG had previous kidney transplantation. Seven patients (10.6%) were transplanted during the observation period. Of the 114 patients who underwent dialysis in the MCA, 11 had previously undergone parathyroidectomy, none occurring during the study period compared to 8 patients in the WFG hospital, with 4 occurring during the study period. Standard dialysate calcium concentration was 1.25 mmol/l and 1.50 mmol/l in the MCA and WFG respectively
While there were significant differences in calcium (uncorrected), phosphate and iPTH between the centers in September 2011, these differences were no longer present in September 2012 (Table 2).
In September 2012, significantly more patients had a dialysate with a calcium concentration of 1.50 mmol/l in the MCA compared to September 2011 (33% vs. 21%, p<0,002), but still considerably lower when compared to the WFG (100%).
At the start of the observation period, significantly more sevelamer was prescribed in the MCA. At the end of the study period, it was just the other way around. Average daily elemental calcium supplementation per patient did not differ significantly between the hospitals, though it was slightly higher in the WFG. On the contrary, within the group of patients who were prescribed calcium-based phosphate binders, the mean dose of calcium was significantly higher in the WFG (1.7 gram/pt/day vs 0.7 gram/pt/day in the MCA in September 2012). This difference may be caused by the fact that there was a more restrictive policy regarding calcium prescription in the MCA as opposed to the WFG. None of the patients in the MCA had more than 1500 mg of elementary calcium per day prescribed. During the study period, elementary calcium from calcium acetate was used significantly more in the MCA (from 128 to 338 mg/p/day, p<0,001) with a simultaneous drop in the use of calcium carbonate (from 401 to 267 mg/p/day). The percentages of patients using either paricalcitol or alfacalcidol was significantly higher in the MCA compared to the Westfriesgasthuis at both time points, but the use of paricalcitol in the MCA diminished significantly during the observation period. Paricalcitol was not prescribed in CKD-MBD management the WFG. Alphacalcidol was prescribed significantly more often in the MCA at both time points. The percentage of patients using magnesium as a phosphate binder and the average daily dose of magnesium also increased significantly from September 2011 in the MCA. Magnesium was not prescribed as a phosphate binder in the Westfriesgasthuis (Table 3).

Table 1: Baseline characteristics expressed in mean with standard deviation, interquartile range (10-90 percentile) and percentages. P-value <0.05 is significant. NS= not significant.
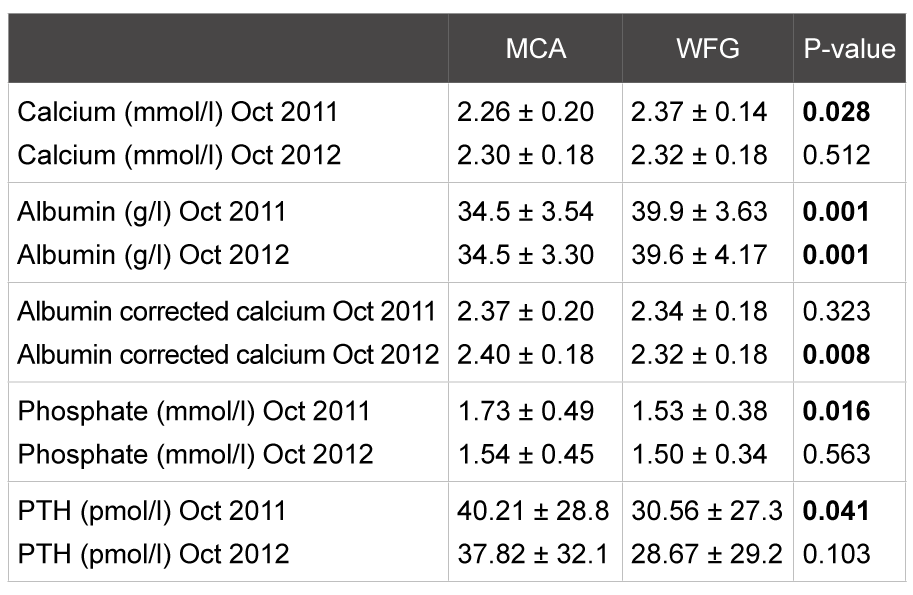
Table 2: Biochemical parameters: mean plus standard deviation and significance (p-value). P<0.05= significant
The total cost of CKD-MBD medication per patient per day in the MCA in September 2011 was 11.34 euro and 4.99 in September 2012. CKDMBD medication cost per patient in the WFG hospital was 4.31 euro in September 2011 and increased to 5.43 euro in September 2012 (Figure 1).
In this observational study, we evaluated differences in the management of CKD-MBD between two centers and the costs associated. The two main differences between the hospitals were in the prescription and use of vitamin D and the management of calcium.
Both the native and active forms of vitamin D were prescribed significantly more often in the MCA. Active vitamin D was supplemented to all patients independent of serum PTH levels in the MCA but only in cases of hyperparathyroidism in the WFG. Several arguments support the use of vitamin D in CKD-MBD management independent of serum PTH levels. Low or deficient levels of vitamin D is considered an independent risk factor for cardiovascular diseases and mortality [6,7]. Insufficiency and deficiency is also prevalent in chronic kidney disease [8-10], even in patients undergoing dialysis and having vitamin D supplements. Active vitamin D supplementation is also associated with improved survival [6,7,11]. Low levels of vitamin D are considered pivotal in the pathogenesis of secondary hyperparathyroidism [8,12,13]. On the other hand, over supplementation with vitamin D may lead to increased episodes of hypercalcemia and hyperphosphatemia, thereby increasing the use of expensive calcium free phosphate binders. Hypervitaminosis D may also lead to increased vascular calcification in CKD patients [14,15]. The combined decrease in prescription of both active vitamin D and calciumfree phosphate binders in the MCA during the study period suggests that the optimal dose of active vitamin D was lower than the dose prescribed in September 2011 in the MCA.
Calcium management was another difference between the hospitals in MBD management. Firstly, in the Westfriesgasthuis only a dialysate with a calcium concentration of 1.50 mmol/l was used. In the MCA most patients had a dialysate with a calcium concentration of 1,25 mmol/l at the start of the observational period. This concentration was primarily chosen to attain a net removal of calcium during dialysis, thereby preventing a socalled “positive calcium balance”. An overall influx of calcium is thought to contribute to increased deposition of calcium phosphate crystals in mainly the tunica media of the arterial wall, leading to stiffening and an increase in cardiovascular risk [2,16]. Indeed it was shown that 626 ± 487 mg calcium per dialysis session can be removed with a dialysate calcium of 1,25 mmol/L [16]. On the other hand, a low dialysate calcium concentration might also increase the risk of sudden cardiac arrest [17]. A higher dialysate concentration may be associated with increased vascular calcification and stiffness [18]. During the observation period, the percentage of patients using a dialysate with a calcium concentration of 1,50 mmol/L Ca significantly increased from 21.1% to 33.3% in the MCA. This was not accompanied by an increased prescription of calciumfree phosphate binders. A possible explanation could be that in September 2011, a number of patients in the MCA had a negative calcium balance. A negative calcium balance is associated with increased parathyroid hormone secretion, leading to secondary hyperparathyroidism and hyperphosphatemia. In September 2012, significantly more patients in the MCA had calcium supplementation although the average dose did not increase.
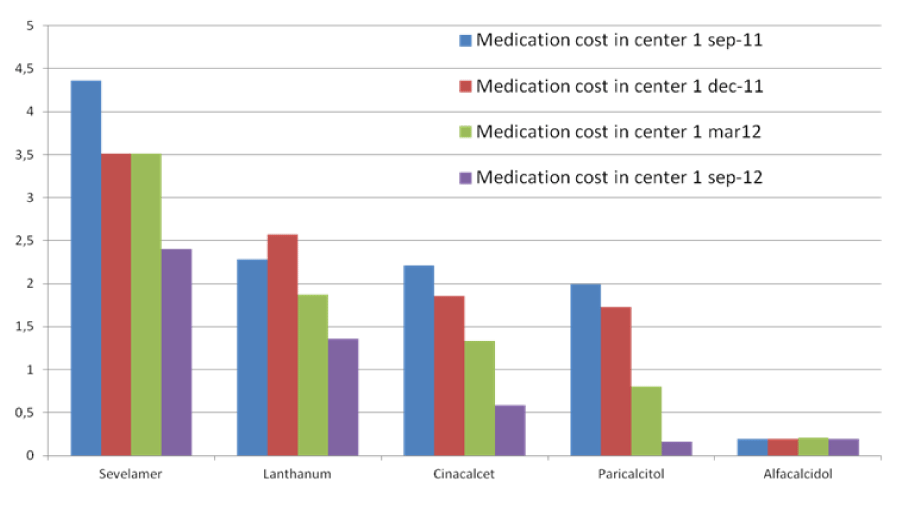
Figure 1: (Center 1=MCA)
Secondly, there was a less restrictive policy with regard to the use of calcium based phosphate binders in the WFG compared to the MCA in September 2011. Within the group of patients that were prescribed calcium based binders the mean dose of elementary calcium was significantly higher in the WFG. KDIGO guidelines 3 advise a limitation (although not specified) in the use of calcium based phosphate binders which are associated with increased arterial calcification and cardiovascular mortality [19-22]. This has resulted in increased use of calcium free phosphate binders which are thought to slow/attenuate this process of arterial calcification. Added benefits of lesser patients hospitalization, pleiotropic effects and positive effects on lipid metabolism and systemic inflammatory response have also been observed with their use [23,24]. However, the only prospective randomized trial that compared calcium based phosphate binders with calcium-free phosphate binders, did not show an increase in mortality in the group treated with up to 2000mg of calcium based phosphate binders [19]. During the observation period, there was a less restrictive policy towards the use of calcium based binders in the MCA although the target for serum calcium remained 2.40 mmol/l. Calcium was also no longer corrected for (falsely low) albumin in the MCA allowing for an increase in the number of patients that received calcium-based phosphate binders.
Between September 2011 and September 2012, there was a significant reduction in medication cost for CKD-MBD management in the MCA. In September 2011 the totals cost of CKD-MBD medication per patient per day in the MCA was 11 euro dropping to 5 euro in September 2012, while the low cost in the WFG remained stable (Figure 2). It is likely that the use of calcium acetate in combination with magnesium has contributed significantly to this result. Firstly, the amount of phosphate bound per mg of calcium is higher in calcium acetate than in calcium carbonate [25]. Secondly, magnesium is a less expensive calcium free phosphate binder than sevelamer or lanthanum. Some additional clinical advantages have been linked to magnesium in CKD [26-28]. The implemented changes in the management of CKD-MBD in the MCA did not lead to deterioration of biochemical parameters. On the contrary, a trend in improvement in phosphate levels was seen.
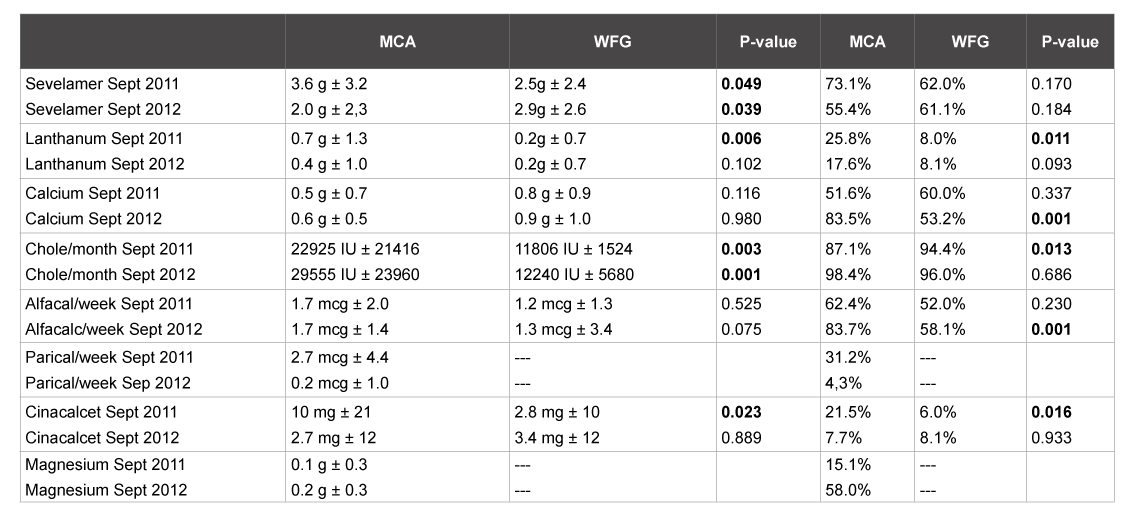
Table 3: Medication expressed in mean (daily/weekly/monthly) plus standard deviation, p value (using non-parametric test) and percentages of patients using the drug. P<0.05 was significant.
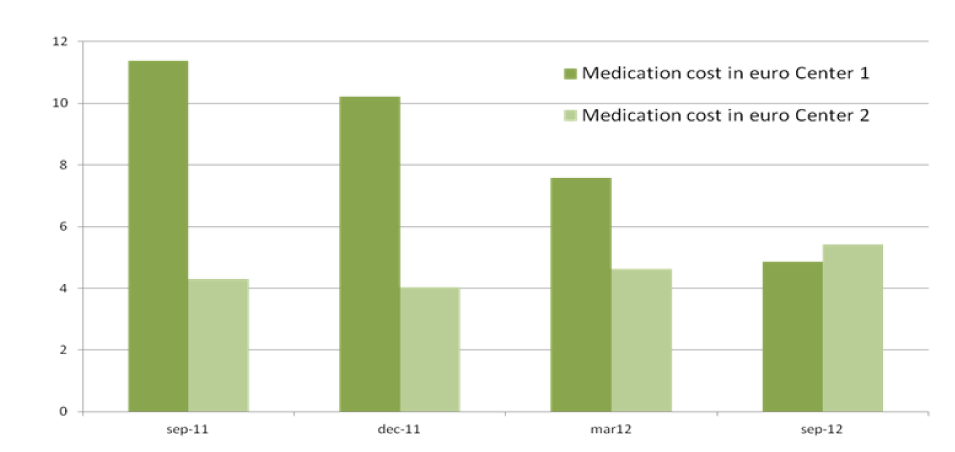
Figure 2: (Center 1=MCA, Center 2= WFG)
Significant differences in residual renal function may have contributed to the differences in CKD-MBD management strategy [29,30]. This study is observational and multiple interventions were done simultaneously, making it difficult to point out or analyze which intervention had the most effects. Also the variables are too many to obtain a safe multivariate approach. Finally, the length of the study is not long enough to evaluate clinical results. The stable or improving biochemical parameters and significant reduction in cost are encouraging. It is also encouraging that age-adjusted mortality rates in both hospitals were lower than National mortality rates and did not differ significantly between the hospitals.
We argue that a combination of the above led to a non-inferiority treatment of CKD-MBD patients with a significant reduction in cost. This observational study highlights the differences that can exist between treatment centers within the KDIGO guidelines and the associated cost that can come with the chosen management strategies. We conclude that comparison between centers in the management of CKD-MBD n the absence of randomized clinical trials may be an effective means of improving care and reducing cost.
Download Provisional PDF Here
Article Type: Research Article
Citation: Jallah BP, Raasveld MHM, van Dam B (2015) Cost Effective Management of CKD-MBD: An Observational Study. Int J Nephrol Kidney Failure 1(1): doi http://dx.doi.org/10.16966/ijnkf.104
Copyright: © 2015 Jallah BP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access