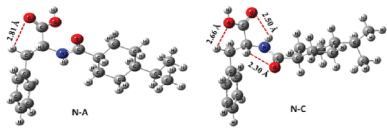
Figure 1: B3LYP/6-31+G (d,p) optimized important conformers of nateglinide in gaseous phase [14].

Mansur Sa’id1* Amina Salihi Bayero2 Usman Lado Ali2
1Department of physics, Northwest University, Kano, Nigeria*Corresponding author: Mansur Sa’id, Department of physics, Northwest University, Kano, Nigeria, Tel: 08065418754; E-mail: mansursaid79@gmail.com
Diabetes is a deficiency in the body’s capability to transmute excess glucose to glycogen. Two classes of antidiabetic drugs were identified and nateglinide is a sub-division of oral antidiabetic drugs belonging to Meglitinides family. Gaussian 09 code which uses density function theory as working principle was used to study the geometric, infrared, Raman, 1H-NMR, 13C-NMR spectrum and density of state of the nateglinide antidiabetic drug using exchange functional B3LYP/6-311G. For the geometry optimization, it was observed that there is the appearance of a new additional bond between C12 and N4 which changes the natural nature of both 1H-NMR and 13C-NMR spectra. For infrared and Raman spectra, functional groups and polarizability were observed. However, the result of the density of state shows the large band gap of 5.46758eV and most of the orbitals were occupied by lower valence band.
Nateglinide; Infrared; 1H-NMR; 13C-NMR; Density of state (DOS); Raman spectra
Diabetes is an imperfection in the body’s ability to metamorphose extra glucose to glycogen. Glucose is the cardinal source of energy to our body. Food digests to form protein, fat or carbohydrate depending on the nature of the food. Carbohydrate (potatoes, rice, corn, fruit, milk etc.) affected blood sugar directly. A diabetic patient can eat carbohydrate in a moderation. Because glucose transferred into the blood and inject energy into the cells. Patient with diabetic diets lacks enough hormone-insulin produce by the β-cells of the pancreas [1].
Pancreas failure to produce sufficient amount of insulin (type 1 Diabetic) for children and young adult resulted in Diabetes. However, defective production of insulin that results in not moving glucose into the cell (type 2 Diabetics) and mostly affected by Adult. The symptoms associated with diabetes include; blurred vision, unexpected thirst, regular urine, slowhealing cuts, miraculous tiredness, erectile dysfunction etc [1].
Diabetes resulted when the blood sugar increased due to inadequate or inefficient insulin release by the pancreas. Blood sugar is then discharged via excretion (urination) governing to cause Glucosuria or glycosuria. This may cause numerous problems such as Cardiovascular diseases, nerves damage and even serious coma etc. patient with diabetic diseases most be given continual treatment to sustain the stability in the blood glucose.
Anti-diabetic drugs are classified thus:
(a) Insulin: mostly use in severe cases of diabetes.
(b) Oral anti-diabetic drugs: conveniently for most adult patients, that are commonly type 2 and oral anti-diabetic drugs include:
(i) Sulphonylurea: they enlarge insulin discharge e.g. Chlorpropamide, Glibenclamides & Gliclazide
(ii) Biguanides: e.g. Metformin.
(iii) Intestinal α-glucosidase inhibitor: they prolong the digestion and assimilation of carbohydrates, and consequently decrease the blood sugar raising after diet. Eg. Acarbose, & Miglitol.
(iv) Meglitinides: it induce early insulin secretion. e.g. Repaglinide & Nateglinide [2,3].
Type 2 diabetics are managed by Nateglinide oral drug popularly known as non-insulin dependent [4]. The drug is a meglitinide past-acting β-cell selective and Potassium Adonosine Triposphate (KATP) blocker for improving glycemic control. Nateglinide is a novel oral glucose regulator. It contains amino-acid that reduce blood sugar level by triggering insulin from the pancreas [5,6].
Nateglinide is directly related to Sulphonylureas (SUR1) and it binds quickly to the SUR1 receptor with low affinity. The prompt link and break makes the drug a unique (Fast-on Fast-off effective). Taking Nateglinide instantly before meal, it will stimulate insulin and provide control for postprandial hyperglycemia. The common action of both Nateglinide and SUR1 are on hypoglycemic such as glibenclamide and glipizide. Clinical comparison on both KATP and peroxisome proliferation active receptor (PPAR) gamma have shown that Nateglinide is more serviceable in attenuating postprandial glucose than any other hypoglycemic. Treatment of diabetics with Nateglinide provide patient with effective improvement for controlling plasma glucose [5].
In this work, the molecular structure of Nateglinide antidiabetic drug was modeled. The conformer with the lowest energy was chosen among the four conformers. Geometry optimization, Infrared, Raman spectrum, Density of state and 1 H & 13C-NMR were computed.
Basically, drugs are designed in such a way that it is activity result from its binding to the receptor as such protein [7]. When ligands bound within the binding moiety, it shows geometric and chemical complementaries [8]. In any drug designed studies, a receptor is regarded as a fairly rigid molecule. While a ligand has a number of degree of freedom, dominantly torsional ones around bonds connecting atoms. Any kinematically allowed substitution of the ligand’s atoms in 3D spatial orientation defines conformation and this conformation predicts the ligand’s internal energy. The lower the energy of conformer the higher the stability [9,10].
The confirmation of a molecule is largely controlled by the number of freely rotatable bond or the degree of freedom (DOF) together with the bond angle, torsional angle and bond length. In reality, only torsional DOFs are regarded because these are the only ones that attain greater variation [7]. In a number of pharmaceutical molecules, the numbers of torsional DOFs are between 3 and 15 [11].
The different conformations result from rotation about the single bond, and the energy needed for the rotation is below 10kcal/mol in most cases [12]. In nateglinide, there are six available rotatable bonds; they are about C1 -C2 , C2 -C3 , C3 -N4 , C5 -C6 , C7 -C8and C3 -C9 bonds [13]. B3LYP optimization of the four reported conformers (N-A, N-B, N-C & N-D) shows that N-A and N-B vary only in –O-C5-C6 -H torsional angle. The torsional angle of N-A and N-B is across a common rotatable bond thus regarded as same. N-C and N-D conformers appeared to be similar at the end of the optimization. Therefore, only two conformers (N-A & N-C) are of significant importance. The relative position of the hydrogen in the alkanoic acid and the torsional angle (-169.17° in N-A & -118.57° in N-C) across C2 - C3 -N4 -C5 are the only differences between the two conformers. The presence of an intermolecular hydrogen bond can be seen between the carbonyl oxygen of –COOH group and amidic hydrogen of –CONH2 (Figure 1) in the optimized geometry (structure) of N-C. This –O-H interaction confers stability to N-C over N-A with an energy of about 1.15kcal/mol lower than N-A. In both gaseous and solvent phases, similar trend was observed in the energy variation of the two conformers at B3LYP/3-21+G(d) level of optimization coupled with energy estimation at B3LYP/6-31+G(d,p) level [14].
Gaussian 09 code [15] which use Density functional theory calculation was performed on the molecular structure of the Nateglinide sources from Ligand Expo (www.http://ligandexpo.rcsb.org). Conformational search was performed on the dihedral angles; C20-C18-C16-H43, C16 -C15 -N4 -C12, and O3 - C17-C15-C14. The conformer with the lowest energy was chosen and optimized using a different basis set of; 3-21G, 6-31G, and 6-311G. From the highest basis set, frequency calculations were performed. Local minima or saddle point (transition state) were the determinant for the measurement of zero point energy. From the result Infrared, Raman and Density of state spectra ware plotted. However, 1H and 13C-NMR calculations on both the structure before and after optimizations were carried out and the result was plotted.
From table 1, it was observed that the conformer C20-C18-C16-H43 has the lowest energy of -2762.448518 eV when compared with others and hence it’s the most stable. However, it was discussed in detail [16] shows the result of relative energy against torsion angle rotated through 60o , and it was found that the lowest energy -415.4805535 eV rotate at 240° and transition state of 400.8615826 eV.
| s/n | Torsional Angle φ | Total energy (a.u.) | Total energy (eV) |
| 1 | C20 -C18 -C16 -H43 |
-1015.180593 | -2762.448518 |
| 2 | C16 -C15 -N4 -C 12 |
-1015.178926 | -2762.443982 |
| 3 | C9 -C6 -C5 -C11 |
-1015.180577 | -2762.448477 |
| 4 | O3 -C17 -C15 -C14 |
-1015.17888 | -2762.443858 |
Table 1: Total energies of conformers of Nateglinide calculate using rB3LYP/3-21G.
As discussed in the [16] it was discovered that there was increased in the bond between Nitrogen N4 and carbon C12 as shown in figure 1. Figure 1a shows the initial geometry of the structure with a single bond between N4 and C12. Also, figure 1b shows the appearance of the additional bond between N4 and C12.This was due to the fact that after optimization, the structure was now in the ground state and hence the energy was the lowest -2777.84622 eV.

Figure 1: B3LYP/6-31+G (d,p) optimized important conformers of nateglinide in gaseous phase [14].
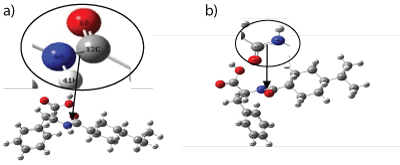
Figure 1a and 1b: Optimized structure of Nateglinide antidiabetic drug obtained: (a) Before Optimization (b) After Optimization.
Infrared and Raman spectra are indispensable apparatus for the lucubration and demonstration of molecular structures (organic or inorganic). Vibes frequencies appear in the dominion of the electromagnetic spectrum at 13333 cm-1 to 50 cm-1 range and beyond are generating a change in the active dipole-moment and vibration of molecules produces polarized electron cloud know as active Raman.
For this paper 144 possible molecular vibration was computed for nonlinear Nateglinide molecule structure using the relation 3N-6 (N= 50).
Figure 2 shows the infrared spectrum of Nateglinide antidiabetic molecule. The spectrum occurs in the frequency region of 4000 cm-1 to 28 cm-1. It was observed that there mono-substituted benzene peaks at around 750 cm-1. Also, C=O peak was observed at 1650 cm-1 intensity of 333.887 D (10-40 esu2 cm2 ). However, there are peaks at range 2921.26 cm-1 to 3147.88 cm-1 with the highest intensity of 122.03 D (10-40 esu2 cm2 ) due to C-H (aromatic) single stretching and OH sharp peak. Finally two peaks appear at 3585.48 cm-1 and 37105.1 cm-1 with frequencies at 13.5589 D(10-40 esu2 cm2 ) and 59.32 D(10-40 esu2 cm2 ) respectively these were due to NH (secondary amine) stretching. The two peaks may result from formation and deformation of hydrogen bond between –NH and neighboring C=O.
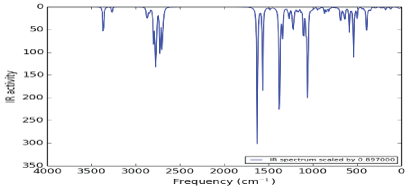
Figure 2: Infrared spectrum of Nateglinide antidiabetic drug.
Figure 3 shows the Raman spectrum produced by Nateglinide antidiabetic molecules. The spectrum was produced in the frequency ranging from 4000 cm-1 to 20 cm-1. It was observed that there are strong symmetric stretching between Carbon and Hydrogen (υ (C-H)) at frequency between 2800-3000cm1 . Between 3300-3500 cm-1, there is medium stretching bond between Nitrogen and Hydrogen (υ (N-H)). Also there, are three weak bond (i.e one asymmetric (δ (CH3 )) and two symmetric (υ (C=O)) and (υ (C=N)) at ranges of frequencies; 1390-1470 cm-1, 1600-1800cm-1 and 1500-16500 cm-1 respectively.
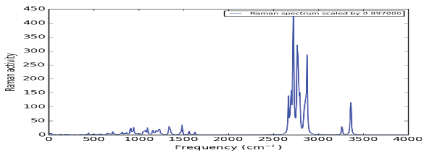
Figure 3: Raman spectrum of Nateglinide antidiabetic drug.
The density of state in a condensed matter gives an account for the possible number of state per interval of energy available occupied. Also, it has been used to widely to study physical properties of matter and essential for determining the carrier concentrations and energy distributions of carriers within the semiconductors. Figure 4 shows the density of state spectrum of Nateglinide antidiabetic drugs. It was observed that between the energy ranges of -20 eV to -15 eV, there is five number of state and highest intensity of 2 eV-1. Between -15 eV to -5 eV there is ten number of states and highest intensity of 4.5 eV-1 . This shows that electrons populations are higher at valence band. Also, the energy gap of 5.46758 eV was measured. However, due to the large band gap, the most of the electrons were at higher valence band and hence little electrons were transferred to the lower conduction band.
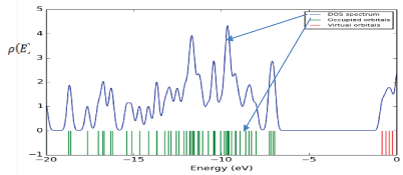
Figure 4: Density of state spectrum of Nateglinide antidiabetic drug.
Figure 5 and 6 shows the predicted 1H-NMR and 13C-NMR structure of Nateglinide antidiabetic drugs respectively. ChemDraw 12 software was used to predict the 1H-NMR and 13C-NMR structure of the original geometry. It was found that the shielding range for 1H-NMR and 13C-NMR were 0 to 12 ppm and 0 to 180 ppm respectively. Also for 1H-NMR there were more Hn at upfield at about 4 ppm and little at downfield. A similar trend was observed for 13C-NMR with more Cn below 60 ppm (upfield) and little at downfield.
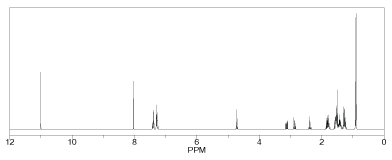
Figure 5: Predicted 1H-NMR before optimization of Nateglinide antidiabetic drug.
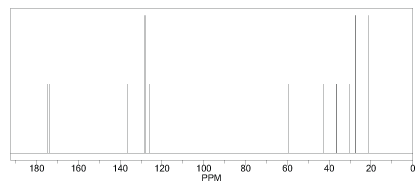
Figure 6: Predicted 13C-NMR before optimization of Nateglinide antidiabetic drug.
Figure 7 shows the 1H-NMR, and it was observed that H45, H46, H48, H49, and H50: are aromatic protons spread over the range 25.39 to 25.86 ppm relative to the rest protons; this indicates that they are relatively equivalent magnetically as they are all within 0.47 ppm. H47: shielded up field less than aromatic/ benzene protons high than other protons. H42: deshielded down to around 27.87 ppm for being attached to the carbon atom directly bonded to nitrogen. H41: deshielded down further to 28.25 ppm for been directly attached to nitrogen which can directly conjugate with neighboring carbonyl group. H43 and H44: methane hydrogens spread over 29.19 to 30.44 ppm and are directly connected to the carbon atom neighboring aromatic ring and hence deshielded. H28, H25, H33, H32, H30, H29, H35, H40, H31 and H34: they are deshielded to a range 30.73 to 31.52 ppm (i.e., they are all spread within 0.89 ppm) they have almost the same magnetic effect.

Figure 7: 1 H-NMR after optimization of Nateglinide antidiabetic drug.
Figure 8 shows the 13C-NMR. It was found that C17 is the most shielded carbon atom despite been doubly bonded to oxygen in one way and single bonded to another oxygen atom in another way thought to be downfield but upfield this largely attributed to C12-N4 junction. In fact, all the unusualities of these NMR spectra both 1 H, 13C, 14N, and 16O obeys the dividend of C12-N4 junction. C12 also shielded relatively to the other C-atoms except for C17. Also, C18 is the most shielded of all the benzene carbon for being the closest to the C12-N4 junction. For C22, C19, C20, C21, and C22 are the remaining carbon atoms of the aromatic ring which showed signal between 54.24 to 56.34 ppm and are relatively upfield and have a relatively similar magnetic effect on the applied field. For C15, C8, C5, C16, and C11: the absorption peaks of these carbons appeared in the range 131.36 to 148.82 downfield. C7, C6, C9, and C10: are downfield carbons of cyclohexane moiety, the other two carbons (C5 &C 8 ) are upfield relative to the aforementioned ones. Lastly, C13 and C14 are carbons showed closed similarity in a magnetic field and hence absorbed in closed δ-value and were the most desheilded carbon atoms of the molecule.
Figure 8: 13C-NMR after optimization of Nateglinide antidiabetic drug.
Nateglinide is belonging to the family of Meglitinides and special antidiabetic drugs for diabetics (type 2). Its effect on the diabetic disease was emphases and the need to improve its chemical manufacturing was also highlighted. Conformational search was performed on the different dihedral angles on the molecular structure. The structure with the lowest conformer was chosen and optimized under different basis sets. The ground state energy of the highest basis set was found to be -2777.84622 eV. However, infrared spectra (IR-spect), Raman spectra (R-spect), Density of State spectrum (DOS) and Nuclear Magnetic Resonance spectra (NMR) studies on the structure were reported.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Sa’id M, Bayero AS, Ali UL (2018) Determination of Infrared, Raman, (1H and 13C)-NMR Spectra and Density of State of Nateglinide Oral Antidiabetic Drug. Int J Nanomed Nanosurg 4(1): dx.doi.org/10.16966/2470-3206.124
Copyright: © 2018 Sa’id M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access