Table 1: Nanoparticles and their applications in dermatology and cosmetology

Tasleem Arif1* Mohammad Adil2
1Assistant Professor, Postgraduate Department of Dermatology, STDs and Leprosy, Jawaharlal Nehru Medical College (JNMC), Aligarh Muslim University (AMU), Aligarh, India*Corresponding author: Tasleem Arif, Assistant Professor, Postgraduate Department of Dermatology, STDs and Leprosy. Jawaharlal Nehru Medical College (JNMC), Aligarh Muslim University (AMU), Aligarh, India, Tel: +919557480345; E-mail: dr_tasleem_arif@yahoo.com
Nanotechnology is the application of the science dealing with the study of small particles with dimensions of less than a hundred nanometers. Nanoparticles, owing to their size, have special physicochemical properties that are distinct from the derived bulk material. These nanoparticles have recently been employed for various diagnostic and therapeutic purposes and are particularly suited for use in the field of dermatology. This article discusses in brief the types of nanoparticles, the current and potential uses of nanoparticles in dermatological and cosmetic medicine. An account of potential hazards of indiscriminate use of nanoparticles has also been added to the review.
Dermatology; Medicine; Nanoparticles; Nanotechnology
Nanoscience, as the name suggests (nanos, Greek=dwarf), is the scientific study of small particles, with at least one dimension in the range of 1 to 100 nanometers. Nanotechnology is the application of this scientific knowledge for various diagnostic and therapeutic purposes [1]. This technology has kick started an exponentially expanding industry, still considered in its infancy by experts, that promises to revolutionize medicine, in terms of diagnosis and treatment, as nanoparticles are believed to be more specific, efficacious, customizable and cost effective. Nanoparticles may show physical and chemical properties quite distinct from similar material with particles of a larger scale, opening the exciting gateway for novel uses of already existing substances [2]. Nanodermatology is the use of nanotechnology in the prevention, diagnosis and treatment of various skin disorders and in the field of cosmetology.
Richard Feynman, considered the father of nanotechnology, was the first to conceptualize that molecules and atoms can be manipulated to form small components, not visible to the unaided eye [3]. The term nanotechnology was given by Professor Norio Taniguchi in 1974, referring to “process of separating, consolidating and deforming materials atom by atom or molecule by molecule” [4]. Nanotechnology has since then emerged as a fast growing field with great interest shown by pharmaceutical industries, medical professionals and patients alike.
The skin is one of the largest organs of the body and provides ample opportunities for nanoparticles to act. However any topical delivery system intended for systemic effects has to traverse the barrier formed by surface lipids, stratum corneum and other epidermal layers to reach the dermis where blood vessels are situated. Substances use one of the three possible pathways to traverse the epithelial barrier. Trans cellular permeation, where substances need to pass through cells of stratum corneum and intercellular lipids [5]; trans-appendageal permeation, where solute passes through hair follicles and sweat ducts [6]; and intercellular permeation where solutes pass tortuously via the extracellular lipids between stratum corneum cells [7]. Particles of size greater than 500 Daltons cannot pass through intact skin [8]. Hair follicles can act as micro-channels and are utilized as a conduit for the entry of such larger particles [9]. It has been demonstrated that particles of size as large as 10 micrometers can penetrate the hair follicle orifice [10]. Nanotechnology aids in drug permeation by releasing active substances at specific sites, increasing stability, ensuring adequate contact, enhancing stability and reducing the need of chemical enhancers [11].
Nanoparticles can be classified on the basis of size, shape structure, physical and chemical properties. Nanoparticles can be spherical with a phospholipid covering, called liposomes or vesicle with a polymeric membrane, termed nanocapsules [12]. Lipid particles in an aqueous base termed solid lipid nanoparticle have hydrating photoprotective properties [13]. Drugs can be conjugated with lipids for increasing drug loading and targeted release [14]. Semipolymeric nanoparticles with a branched tree like structure are called dendrimers, while those packed in a cube like shape are cubosomes. Fullerenes are carbon atoms arranged in the form of a hollow tube, sphere or ellipse [15]. Nanocrystal is the crystalline arrangement of 10-400 nm size particles used for delivering poorly soluble drugs [16]. Gold and silver nanoparticles are utilized for strong antiseptic properties. Nonionic surfactant vesicles called niosomes have high penetration and stability [17]. Microsponges are microporous beads that have controlled drug releasing property, particularly responding to rubbing, temperature, pH and moisture [18]. Virosomes are viral proteins in liposomes and utilized in vaccines [4].
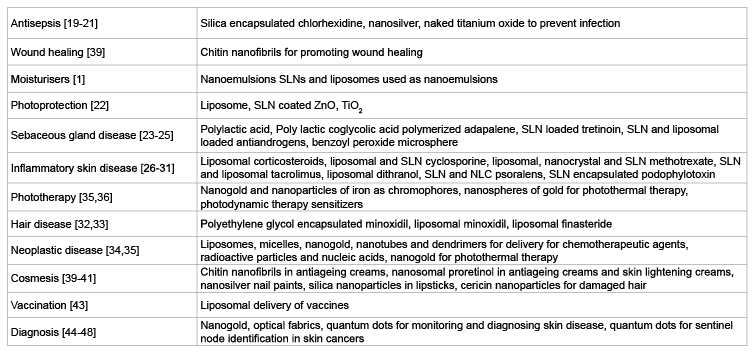
The various uses of nanotechnology in dermatology and cosmetology include (Table 1):

Table 1: Nanoparticles and their applications in dermatology and cosmetology
A large population is being increasingly exposed to nanoparticles, with percutaneous entry, inhalation and ingestion being the common routes of exposure [1,49]. The skin is a large organ and acts as an important portal of entry of nanoparticles; the penetration greatly increases when the skin barrier is disrupted due to wounds or some dermatitis, allowing larger molecules to pass through [50]. The respiratory route is involved during the use of aerosolized cosmetic products like perfumes or during production of such products. Entry into brain via olfactory nerves after inhalation has been documented [51]. Cosmetics like lip colour and lip gloss may gain entry in the body after ingestion. The toxicity of nanoparticles has been explained to be due to their small size, leading to exponential increase in surface area to volume ratio [52]. Other determinants of toxicity include chemical composition, charge, concentration, surface coating, shape and solubility. Toxicity may occur when the host has defective excretory mechanisms, best exemplified by the occurrence of nephrogenic systemic fibrosis in renal failure patients due to accumulation of gadolinium [53]. Sunscreens with nanoparticles of oxides of zinc and titanium accumulate in stratum corneum and their application to skin with abnormal barrier seems risky [1,54]. Titanium oxide generates free radicals on UV exposure and can lead to DNA damage and chromosomal mutations apart from damage to cell constituents [55]. Several in vivo studies regarding safety of nanoparticles in sunscreens have been done with no toxic effects as these particles are not able to penetrate the intact skin barrier [56,57]. Carbon nanotubes have been reported to cause granuloma in laboratory animals [58]. Quantum dots nanoparticles have the potential to penetrate epidermal keratinocytes of neonates and induce inflammation [59]. Concerns are expressed about nanosilver particles after it was demonstrated that the concentration that is toxic to bacteria also damages fibroblasts and keratinocytes [60,61]. Cosmetics with nanoparticles have the potential to induce foreign body granuloma and granulomatous cheilitis [62]. Nanoparticles are highly active particles and have the potential to act as allergens or haptens by reacting with proteins, forming a complex with MHC embedded selfpeptides, T cell recognition and an immune response [10,63]. Teratogenic effects of metal oxides have been shown in human fetal lung fibroblasts in in-vitro studies [64], cranial nerves and genital system in murine models [65] and carbon nanoparticles have shown to decrease sperm counts in murine models [66]. Nanoparticles are released in water, air, soil and food during manufacture and after use by consumers, also posing potential hazards to the environment [67].
Nanotechnology is a new branch of science that is believed to have a great potential for diagnostic and therapeutic purpose in the medical field. Dermatology and cosmetology is particularly more likely to benefit from the immense promise it holds. However, certain concerns regarding the safety of these particles need to be addressed before it is employed on a greater scale.
The authors declare that they have no competing interests.
Download Provisional PDF Here
Article Type: Review Article
Citation: Arif T, Adil M (2016) NanotechnologyDermatological Perspective. Int J Nanomed Nanosurg 2(2): doi http://dx.doi.org/10.16966/2470- 3206.112
Copyright: © 2016 Arif T, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access