Introduction
Quantum dot nanoparticles (QDs) are emerging as a new contrast agent
that can be employed in the biological system due to their potential use in
imaging and therapeutic applications [1,2]. The unique optical properties
of QDs for in vivo imaging include: a high absorption coefficient, a high
fluorescence quantum yield, and high resistance to photo bleaching. More
importantly, the broad absorption and narrow emission spectra of QDs
make them suitable for simultaneous multiplex imaging [3]. However,
the incorporation of QDs into biological systems often requires strategies
for the manipulation of the ligands bound to the surface of the QDs in
order to make them water-soluble [4].
The usefulness of QDs in biomedical applications requires that they
enter the body and contact tissues and cells directly. This perforce requires
knowledge of their biocompatibility. QDs are considered biocompatible
that is, compatible with living tissues or a living system when they are
neither toxic nor injurious or physiologically reactive [5]. By the same
token, QDs are considered toxic when the particles adversely affect the
normal physiology and/or directly alter the normal structure of human
and animal organs and tissues [6,7].
Scientific knowledge regarding the cell-interaction mechanisms of
QDs has grown in recent years. Previous results strongly suggest that
surface coatings can improve cytocompatibility and consequently,
decrease toxicity. Some studies have shown that long-term exposure
of surface coated QDs to their bio environment can destabilize the
binding strength of the surface molecules, which in turn can result in
unprotected QDs inside or outside the cells [8,9]. Therefore, the stability
and binding strength of surface molecules over the QD surface define the
biocompatibility of the QDs and hence, their toxicity. Currently, polymers
with excellent biocompatibility and low toxicity are being successfully
and widely employed to modify the surface of QDs and engineer
biocompatible QDs composites for a variety of medical and biological
applications [10,11].
We recently reported the synthesis of size 3 nm QDs with an increased
green light emitting capacity and coated them with sugar polymers,
concluding they can be potentially used in the field of bioimaging [12,13].
Maltodextrin capped cadmium sulfide (CdS-MDx) QDs have produced
distinct dose-dependent effects in vitro and in vivo; in vivo effects, however,
are unknown. In this study, maltodextrin capped cadmium sulfide (CdSMDx)
QDs were subjected to a biocompatibility analysis. In order to
determine the biocompatibility of CdS-MDx QDs, we histopatologically
examined fluorescence emission in tissue from rodents receiving a daily
dose of nanoparticles during either 5 or 15 days. Therefore, the aim of
the present study was to evaluate the biocompatibility of CdS-MDx QDs
in vivo.
Material and Methods
Analysis of in vivo biodistribution and biocompatibility of CdS-MDx QDs
Bright green emitting CdS-MDx QDs that were 3 nm in size were
synthesized as previously described [13]. The Wistar rat was selected as
the model for the biocompatibility study of CdS-MDx QDs. All animals
were kept in an animal house for 12 hours day/night cycle for 2 months.
All animals were kept in hygienic and animal-friendly conditions, housed
in a temperature and humidity controlled environment, and allowed
food (Standard Purina Chow Diet, Mexico) and water ad libitum. The
experiments were conducted in accordance with the Guide for the Care
and Use for Laboratory Animals [14].
Twenty four healthy Wistar rats (8-10 weeks old) were selected
randomly and divided into three groups: in one group, 8 animals were
treated with a daily i.p. dose of CdS-MDx QDs in 300 µL PBS at 200 µg/
Kg body weight for 5 days; in the second group, 8 animals were treated
with a daily dose of CdS-MDx QDs at 200 µg/Kg body weight i.p., during
15 days, in 300 µL PBS. The control group (8 animals) was injected with
300 µL PBS. Animals were observed for signs of toxicity. Signs recorded
during acute toxicity included: motor activity, anesthesia, tremor, arching,
and rolling, clonic convulsions, ptosis, tonic extension, lacrimation,
exophthalmos, pilo-erection, salivation, depression, ataxia, sedation,
hypnosis, cyanosis and analgesia. Behaviour parameters, death, the
weight, and the amount of water and feed were analyzed. Toxicity indices
consisted of daily clinical observations, body weight, food consumption,
clinical pathology, organ weights, and histopathology.
After treatment, animals were fasted overnight and blood samples
were obtained from the heart following anesthesia with ether. Tissues
collected at necropsy were preserved in 10% neutral-buffered formalin
fixative and were processed for routine histological examination. For
fluorescence analysis, tissue samples were stained with H&E stain. Serum
was separated by centrifugation at 3000 rpm for 15 min. Biochemical
parameters of serum enzyme activities of alanine aminotransferase
(ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP),
glucose, cholesterol, triglyceride (TG), urea, creatinine and uric acid from
animals that received multiple doses of CdS-MDx QDs were measured
using a commercial reagent kit (ELITech, Mexico).
Statistical analysis
The data were represented as the mean ± SD of 3 independent
experiments. The data was statistically analyzed using the SPSS 10.0
software (SPSS Inc., Chicago, Ill., USA), the t-test, and ANOVA.
Differences were considered significant if the P- value was less than 0.05.
Results
In vivo biocompatibility of CdS-Dx QDs fluorescence microscopy
In order to obtain precise data regarding the biocompatibility of
CdS-MDx QDs in rodent tissues, we treated rats with CdS-MDx QDs
at 200 µg/Kg during either 5 or 15 days. The unstained tissue samples
were observed under a fluorescence microscope and CdS-MDx QDs
were identified because of their bright green light. The unstained tissue
samples were analyzed to detect tissue distribution and accumulation. A
histopathological analysis of stained tissue was also undertaken in order to
monitor their toxicity and see if CdS-MDx QDs were biocompatible or if
they induced structural changes. Figures 1 and 2 shows the representative
fluorescence images of the biodistribution of CdS-MDx QDs in intestine,
lung, spleen, heart, kidney, liver, brain, and skeletal muscle from female
rats. Figures 3 and 4 show representative fluorescence images of the bio
distribution of CdS-MDx QDs in intestine, lung, spleen, heart, kidney
liver, brain and testis from male rats. The visualization of differing
fluorescence intensity due to the presence of CdS-MDx QDs in each
tissue was evident in both sexes. In intestine, the CdS-MDx QDs was
more distributed in the adventitia, lamina propria and lamina epithelialis.
In females, fluorescence in these tissues was more evident at 5 days.
In the case of males, however, fluorescence intensity was higher at 15
days. In the lung, evident fluorescence surrounded the blood vessels,
bronchioles (smooth muscle, submucose and cartilage) and alveolar sacs
(luminal alveolar epithelium, smooth muscle and basement membrane);
no significant changes in the intensity of fluorescence were observed in
either sex. In the spleen, fluorescence intensity was high when rats were
treated with CdS-MDx QDs for 5 days. The QDs were observed mainly in
red pulp and, to a lesser extent, in white pulp. After 15 days of exposure,
the CdS-MDx QDs were observed in the manti zone of the follicle and
in the marginal zone of the follicle in female rats, whereas in males the
CdS-MDx QDs were mainly found in red pulp; no significant changes
in the intensity of fluorescence were observed in either sex. In the heart,
CdS-MDx QDs were found in the myocardium (muscle fibers) and were
less evident at 5 days of treatment in female rats.
The kidney showed more fluorescence intensity than any other organ.
This was detected mainly in the proximal and distal convoluted tubule and,
to a lesser degree, in the glomeruli; the bright green emitting was more
intense in female rats. The liver also showed high fluorescence intensity,
mostly around the hepatic portal venule, the branch of the hepatic artery,
the bile duct and, to a lesser extent, the hepatic parenchyma. The bright
green emitting was more intense in male rats.
CdS-MDx QDs evidently crossed the blood-brain barrier and bloodtestis
barrier because we detected fluorescence in brain and testis.
Fluorescence was present in all layers of the cerebral cortex, but it was
higher in the molecular layer in females, and in the external granular layer
and the ganglionar layer in males. The testis showed intense fluorescence
in Leydig cells and reduced intensity in the seminiferous tubule, as well
as in the spermatocytes’ zone. A high uptake of CdS- MDx QDs was
observed in skeletal muscle, and the lowest degree of fluorescence was
detected in the thymus (data not shown). It is interesting to note that,
in most of the tissues, bright green emitting was higher after 15 days of
treatment. No significant changes were observed in either female or male rats.
The histopathological analysis did not reveal any alterations in all
studied tissues after 5 and 15 days of exposure to CdS-MDx QDs (Figures
5 and 6). Analyzed biochemistry parameters did not show alterations
when compared with male rats in the control group (Table 1); however,
an important increase (100%) in serum triglycerides levels was observed
in female rats (p<0.05) (Table 2).
Discussion
Quantum dots have received much attention recently given their
promising role in bioimaging [15,16]. However, the use of QDs for
biological and clinical applications still has serious limitations because
there is not enough data regarding their biocompatibility in vivo, and
this has led to concerns regarding their safety [17,18]. The present study
shows evidence of the biocompatibility of CdS-MDx QDs in rodents, as
well as the high quality of the images that can be obtained using the CdSMDx
QDs.
The biocompatibility of QDs is one of the most important
requirements for their application in bioimaging [19]. In order to
make QDs more biocompatible with their biological environment,
these need to be made hydrophylic. It is therefore crucial that they
be rendered water-soluble through the modification of their surface.
The stability of QDs in water can be obtained by either a complete
ligand exchange procedure, or through steric stabilization where the
native hydrophobic surface is coated with amphiphilic molecules,
like polymers [20,21]. The present study used maltodextrin coated
cadmium sulphide quantum dots which, as previously reported, have
a size of 3 nm [13]. Since maltodextrin has been found to be safe
and non-toxic, we have used it as capping agent in the synthesis
of CdS QDs. Maltodextrin is considered a good coating material
because it exhibits low viscosity and also shows good water solubility.
Pharmaceutically, it is used as a diluent in tablets and as a coating
material in microencapsulation [22]. The development of a new type
of quantum dot coated with certain sugar molecules might facilitate the
attraction to receptors in specific tissues and organs [23].
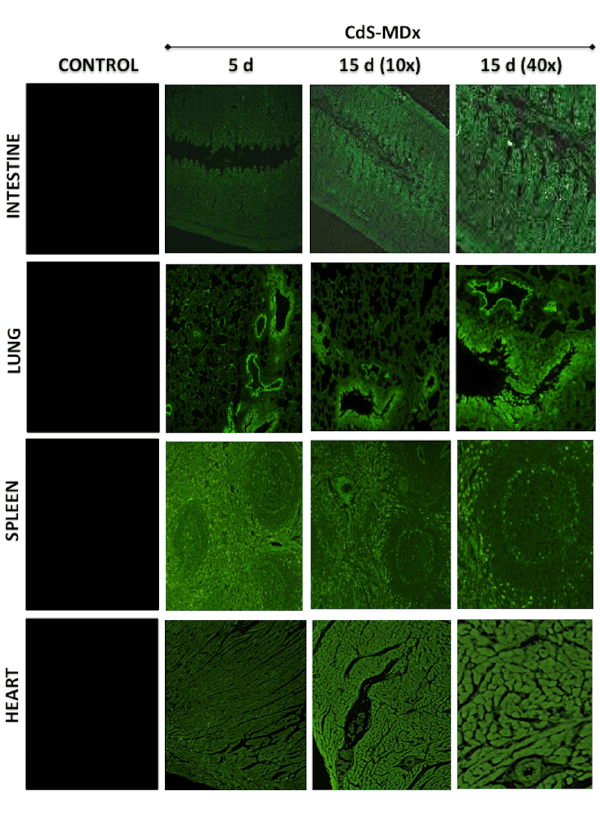
Figure 1: Fluorescence microscopy images showing the distribution and localization of CdS-MDx QDs in tissues from rats. The images belong
to the intestine, lung, spleen and heart of female rats treated with 200 µg/Kg daily i.p., for 5 and 15 days. The left column shows tissues from
the control group observed under fluorescence microscopy. The distribution and localization of CdS-MDx QDs was identified by a bright green
imaging in the analyzed tissues. There is an evident increase in fluorescence in the intestine and heart at 15 days.
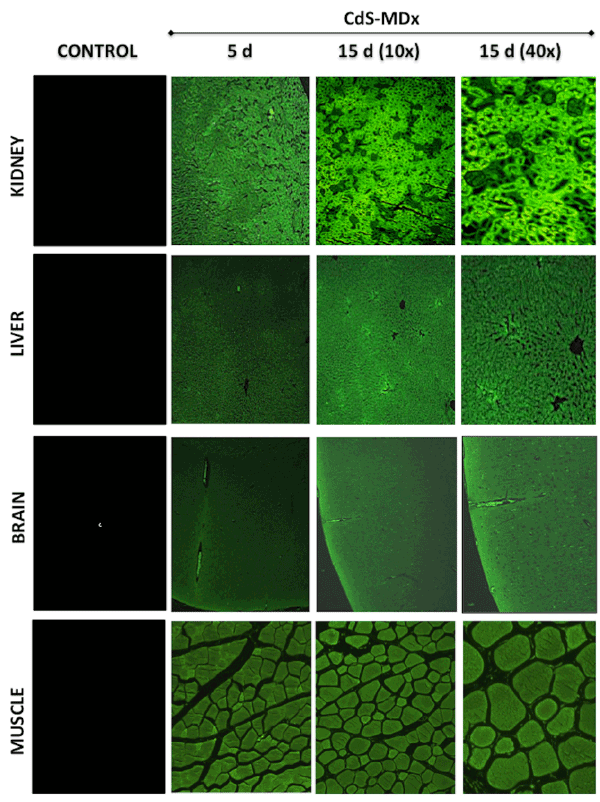
Figure 2: Fluorescence microscopy images showing the distribution and localization of CdS-MDx QDs in tissues from rats. The images belong to the
kidney, liver, brain and muscle of female rats treated with 200 µg/Kg daily i.p., for 5 and 15 days. The left column shows tissues from the control group
observed under fluorescence microscopy. The distribution and localization of CdS-MDx QDs was identified by a bright green imaging in the analyses
tissues. There is an evident increase in fluorescence in all organs after 15 days of treatment.
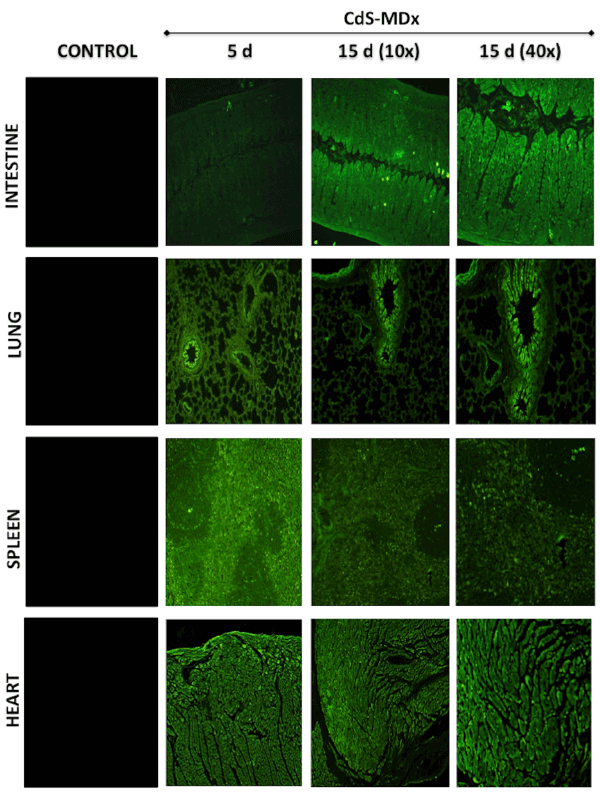
Figure 3: Fluorescence microscopy images showing the distribution and localization of CdS-MDx QDs in tissues from rats. The images belong to
the intestine, lung, spleen and heart of male rats treated with 200 µg/Kg daily i.p., for 5 and 15 days. The left column shows tissues from the control
group observed under fluorescence microscopy. The distribution and localization of CdS-MDx QDs was identified by a bright green imaging in the
analyzed tissues.
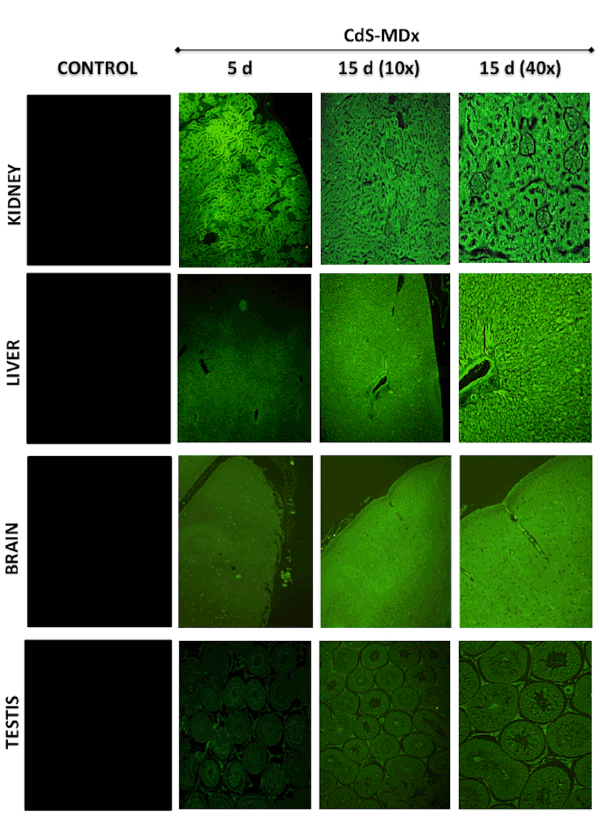
Figure 4: Fluorescence microscopy images showing the distribution and localization of CdS-MDx QDs in tissues from rats. The images belong to
kidney, liver, brain and testis of male rats treated with 200 µg/Kg daily i.p., for 5 and 15 days. The left column shows tissues from the control group
observed under fluorescence microscopy. The distribution and localization of CdS-MDx QDs was identified by a bright green imaging in the analyzed
tissues. There was an evident increase in fluorescence in the kidney, liver and brain after 15 days of treatment.
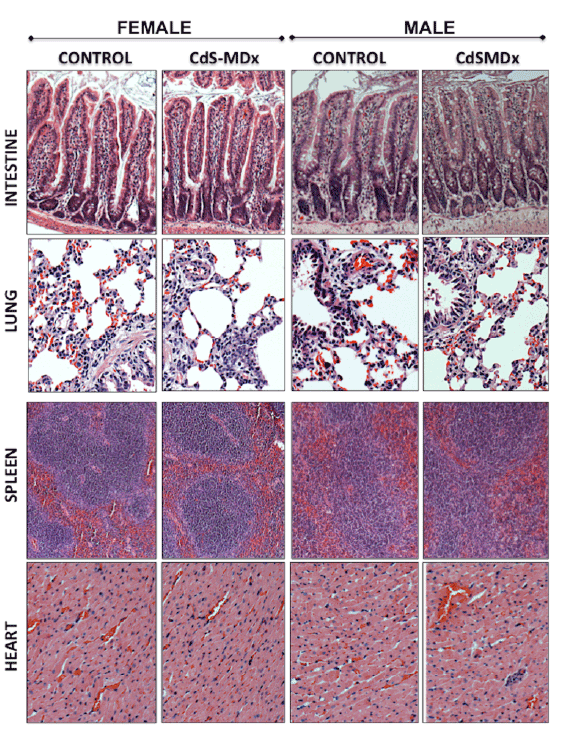
Figure 5: Representative light microscopy image of tissues from rats treated with CdS-MDx QDs. The images belong to the intestine, lung, spleen
and heart from male and female rats treated with 200 µg/Kg daily, i.p., for 15 days. Our analysis shows that the organs do not exhibit signs of toxicity.
The integrity of tissues was analyzed after staining with Hematoxilin & Eosin (Magnification 10X).
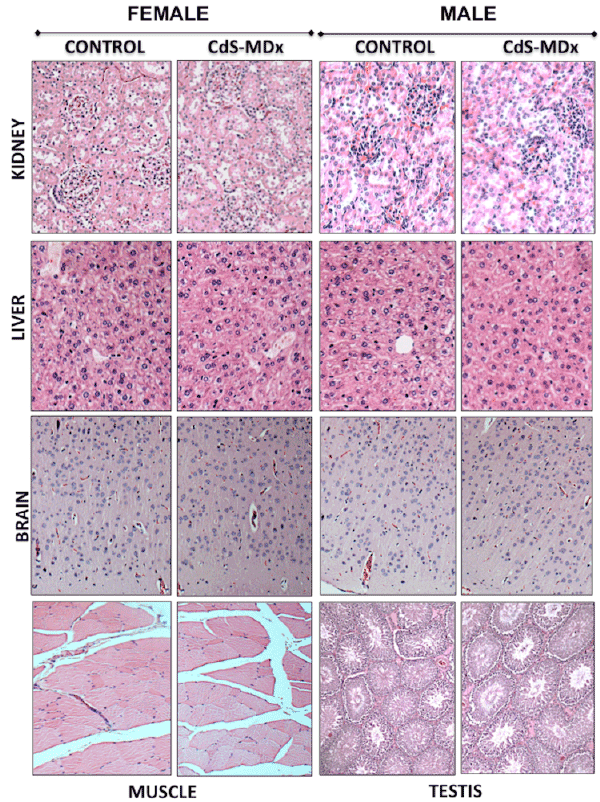
Figure 6: Representative light microscopy image of tissues from rats treated with CdS-MDx QDs. The images belong to the kidney, liver, brain, muscle
and testis of rats treated with 200 µg/Kg, i.p., and daily for 15 days. Our analysis shows that the organs do not exhibit signs of toxicity. The integrity of
tissues was analyzed after staining with Hematoxilin & Eosin (Magnification 10X).
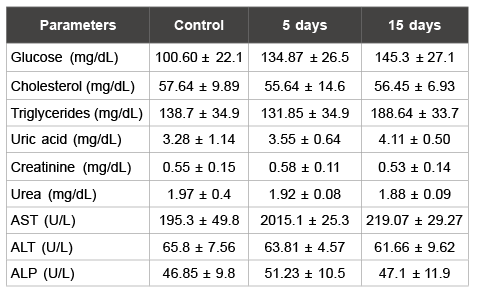
Table 1: Biochemistry parameters analysed in male rats
Data are means ± S.D.
*p<0.05 as compared with control group
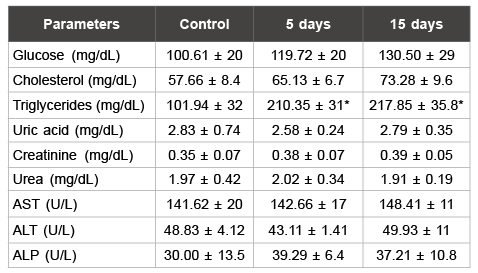
Table 2: Biochemistry parameters analyzed in female rats
Data are means ± S.D.
*p<0.05 as compared with control group
However, the synthesis of each individual type of QD determines its
own unique physicochemical properties, which in turn determines its
potential toxicity or lack thereof [23,24]. In addition, the complexity of the
biological environment in a complete organism means that nanoparticles
observed in vitro conditions may yield entirely different effects in vivo.
This study evaluates the biocompatibility of CdS-MDx QDs in male and
female rodents. Previous reports by this group have shown that CdSMDx
QDs were able to induce cell death by apoptosis and necrosis, their
embryo toxicity, in a dose dependent manner, was also observed in in
vitro and in vivo conditions [13]. Our present results demonstrate that the
administration of 200 µg/Kg CdS-MDx QDs to rodents during 5 or 15
days was biocompatible and non-toxic.
Although studies are limited, the distribution of QDs across tissue/
organs seems to be multi factorial, depending on QD size, QD core-shell
components, and the bioactivity of conjugated or otherwise attached
functional groups [25]. Size alone can markedly affect distribution
kinetics [26]. Present results show that, after intraperitoneal injection,
CdS-MDx QDs were distributed across all studied tissues. This suggests
that the size of CdS-MDx QDs allowed their access into tissues and, like
in the in vitro assay, there was cellular uptake of QDs in all tissues. QDs
lacking specialized functional groups or specificity have been shown to
incorporate into a variety of cell types via endocytic mechanisms, both
in vivo and in vitro conditions [27]. An interesting find was the detection
of bright green emitting in brain and testis, meaning that CdS-MDx
QDs were able to cross the blood brain barrier (BBB) and the blood
testicular barrier. Although previous studies on the biodistribution of NPs
demonstrated their presence in the brain [28,29], no detailed analyses have
been conducted on how frequently QDs are introduced into this organ.
However, present preliminary results offer a basis for the development of
novel CdS-MDx QDs with targeting molecules and drugs, which could
enhance drug delivery efficacy across the BBB and facilitate the uptake
of the QD-drugs into the brain.
Although few in vivo studies exist, these suggest that QDs may
accumulate in a variety of organs and tissues [30,31]. Nanoparticles are
commonly sequestered by phagocytic monocytes and macrophages from
spleen and liver [32], and most of the in vivo biodistribution studies of
nanoparticles have shown the accum ulation of QDs in liver, spleen and
kidney [33]. Present results evidence CdS-MDx QDs are indeed present in
those organs. However, the pattern of biodistribution and accumulation of
CdS-MDx QDs in other organs was different, even considering exposure
time. It has been reported that QD surface coating can govern serum
lifetime and pattern of deposition in vivo [34,35]. Now it is clear that
QD ADME properties and toxicity depend on multiple factors derived
from both inherent physicochemical properties as well as environmental
conditions. Currently, there are no studies comparing the effect of QDs in
both sexes. Our findings did not reveal any differences in the CdS-MDx
QDs’ pattern of distribution in female and male rats.
Safety concerns regarding inorganic nanoparticles are a key factor
in their clinical application. In fact, substantial in vivo studies have
documented that QDs can pose several forms of toxicity, including
nephrotoxicity, hepatic damage, reproductive toxicity and hematological
abnormality [36,37]. Our histopahological analysis of stained tissues
showed that CdS-MDx QDs did not produce morphological changes
in the structure of any of the tissues analyzed, nor were they correlated
to any kind of abnormality in the biochemical analysis. The increase in
serum triglycerides levels in female rats could be due to sex-specific
differences in lipid and glucose metabolism [38]. It has been reported
that estrogens display a high free fatty acids uptake and induce a high
rate of triglyceride synthesis in subcutaneous adipose tissue, as well
as an increase in the serum levels of triglycerides [39,40]. However,
additional studies are needed to clarify the effects of CdS-MDx QDs
on lipid metabolism in females.
Conclusion
Present results demonstrated that, when administered to rodents,
CdS-MDx QDs were biocompatible and non-toxic. The pattern of
biodistribution and accumulation of CdS-MDx QDs was different for all
organs and exposure times, but not differences between sexes were found.
CdS-MDx QDs may warrant further evaluation because they appear to be
promising nanomaterials that can be used in biomedicine for bioimaging.
Acknowledgement
The authors wish to thank to Q.F.B. Xochitl Alvarado Affantranger
from the National Laboratory of Advanced Microscopy at UNAM for her
technical assistance.









