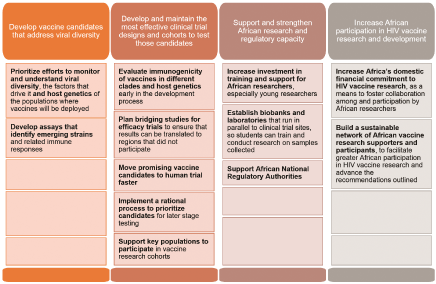
Figure 1: 12 Key Recommendations from the Kigali Working Group on Pan-African HIV Vaccine Development


Amapola Manrique1* Gabriella Scarlatti1 William Snow1 Sabin Nsanzimana1 Pontiano Kaleebu2
1Global HIV Vaccine Enterprise, New York, USA*Corresponding author: Amapola Manrique, Global HIV Vaccine Enterprise, 64 Beaver Street, # 352, New York, NY 10004, United States of America, Tel: +1 212-461-3692; E-mail: Amapola.manrique@gmail.com
Introduction: Africa’s centrality to the global HIV epidemic is well known. Less often discussed are the specific scientific, organizational and regulatory challenges of developing a safe and broadly effective HIV vaccine for the continent where such a tool is most needed.
Discussion: New efforts are underway to develop vaccines that will address the extraordinary viral diversity that characterizes HIV on the African continent; advance more effective clinical trial designs; address regulatory barriers that slow scientific progress; and more effectively engage African researchers and institutions as full partners in the continent’s HIV vaccine development and testing efforts.
In this commentary, the authors summarize recommendations, grounded in these recent efforts, that have emerged from a series of meetings, most recently during the HIV Research for Prevention (HIVR4P) conference in October 2016.The recommendations include a four-pillar strategy, developed at the 2015 Kigali meeting of researchers, regulators, funders and advocates convened by The Global HIV Vaccine Enterprise in collaboration with key stake holders, to overcome the most pressing scientific and organizational roadblocks to developing effective HIV vaccines for Africa, as well as steps that have been taken since then.
Conclusions: The ongoing efforts of this group, including this commentary, aim to bring new thinking to the fore in efforts to overcome longstanding obstacles to the development of effective HIV vaccines for Africa, and to ensure that no country or population is left behind in the search for an effective vaccine.
HIV vaccine; African participation in research; Global HIV vaccine enterprise; Virtual network; Viral diversity; Cohorts; Capacity; Regulatory
Any successful strategy to end the global AIDS epidemic must have at its heart the development of a vaccine that will be safe and highly effective across the entire region most affected by HIV–sub-Saharan Africa. Although Eastern and Southern Africa contain just 5% of the world’s population; those regions are also home to two thirds of the global population of people living with HIV. Sub-Saharan Africa continues to be the epicenter of the epidemic, with 66% of the world’s new infections, and 86% of new infections in children in 2014 [1].
Developing an HIV vaccine that will be broadly effective across Africa is a high priority and a tremendous scientific and logistical challenge. Factors that complicate the search for that elusive vaccine include the significant and potentially increasing diversity of HIV across the continent; challenges associated with supporting the full engagement and maximizing the capacity of African researchers and institutions; the complexities of organizing clinical trials with thousands of individuals at high risk of HIV infection including marginalized groups; and similar, but imperfectly aligned, incentives and priorities.
The Global HIV Vaccine Enterprise (Enterprise) together with its African partners organized a meeting of 60 researchers, regulators, funders and advocates 16-17 March 2015 in Kigali, Rwanda to propose a Pan-African HIV Vaccine Development Agenda to ensure that no country, population or clade is left behind in this effort [2]. This meeting built on discussions at other meetings such as the USAID consultation held in Nairobi, Kenya in May 2014. Here, we outline the group’s fourpillar strategy to support development of effective HIV vaccines for Africa. (Figure 1).

Figure 1: 12 Key Recommendations from the Kigali Working Group on Pan-African HIV Vaccine Development
The extraordinary genetic diversity of HIV-1 strains worldwide is arguably the greatest challenge in HIV vaccine development. That challenge is particularly acute in Africa, where many HIV-1 subtypes and recombinants circulate, and where viral diversity appears to be increasing. Currently, most sequences analyzed were sampled in areas where infections are mostly of the same type (subtype B in North America or Western Europe, subtype C in Southern Africa, CRF1-AE in Thailand) limiting ability to extrapolate those results to regions that are more diverse. HIV-1 is very prone to recombine but precise estimates of the proportion of recombinant strains among all circulating strains are still lacking. There is no doubt that, as HIV-1 global diversity keeps expanding over time, the proportion of recombinant forms is relentlessly increasing, leading to second generation recombinant viruses with unknown biological properties [3]. These viruses that are presently circulating in the region might represent a source of future global multi-drug resistant or vaccine evasion re-emergence events. The understanding is that further analyses are needed to better understand the complexity of the epidemic in areas where multiple clades and CRFs are circulating, as in East Africa (subtypes A1, C and D) or West Africa (subtype G and CRF02_AG).
This tremendous viral diversity across Africa, including the appearance of new recombinant strains [4-6] along with viral fitness dynamics during transmission that are also likely to impact vaccine effectiveness [7], suggests that single clade or consensus vaccines are unlikely to produce a significant impact in many regions of the continent. In response, researchers are developing approaches to broaden the potential impact of a candidate vaccine by: maximizing the key epitopes represented in their candidates; producing envelope proteins with native configurations or molecular mimics of the native structure; infusing broadly neutralizing antibodies; and using mosaic immunogen design to induce more broad humoral and cellular responses [8-15].
Advancing HIV vaccine development for Africa will require a deeper understanding of viral diversity on the continent, the factors that drive that diversity and its implications for pathogenesis and vaccine design [16]. Continuous monitoring of viral evolution and emerging recombinant forms, and improved assays to identify emerging viruses and related immune responses will be essential.
Evaluating the immunogenicity of candidate vaccines in different African regions earlier in the vaccine design process will be critical to improving our understanding of how these vaccines might work in different real-world populations. Designing trials to cover different clades and host genetics will also generate essential information on the breadth of immune responses.
Bridging studies should be planned to ensure that data on advanced candidates include regions that do not participate in a successful efficacy trial. Candidates such as the pox-protein HIV vaccine candidate by the Pox-Protein Public-Private Partnership (P5) [17], a follow up on a candidate that showed moderate protection in Thailand [18], designed with targeted coverage must consider the extent to which their results can be translated to populations and clades not included in the on-going and planned trials.
Overall, there is large agreement that vaccine candidates should be moved to human studies faster, and through a rational process. The immune space concept [19,20], developed by the Enterprise and an ad hoc group of clinical trials collaborators, provides a useful tool to guide discussions of how vaccine products differ to help prioritize late stage efficacy trials. This tool-a list of assays recommended to be performed in every phase I/II trial-provides a standardized approach by which the character, level and durability of immune responses elicited in early human trials by a candidate vaccine can be described.
Developing highly sophisticated vaccine candidates is pointless without an understanding of who is at risk and needs the vaccine most. Studies to engage and understand how risk in key populations differs by locale are complex, expensive, labor intensive, and central to a vibrant African vaccine research effort.
An increasing number of dedicated researchers have developed innovative approaches to providing comprehensive HIV prevention and treatment services to key populations, making participation in vaccine trials a mutually-beneficial experience [21,22]. These experiences should be studied and replicated or adapted in various settings across the continent, to ensure that the stigma, discrimination, and criminalization faced by high-incidence populations such as female sex workers and men who have sex with men are addressed in many African settings.
Perhaps the most effective way, however, to maintain strong vaccine cohorts is to ensure they remain prepared and active at all times through feasibility or simulation studies for example or by making them available to other intervention studies, and ultimately by creating a steady pipeline of strong vaccine candidates to be tested in these cohorts. These, of course can only be achieved with continuous funding and stakeholder engagement.
Even with general agreement that African researchers should be integral members of the clinical trial team, in too many cases African researchers are still more likely to be involved solely in seeing volunteers and processing and shipping samples rather than in protocol development, trial design, immunogenicity studies or research infrastructure development.
While many local and international training programs exist, increased support for continued mentorship, and appropriate research and networking infrastructure (including bio banks and laboratories) is needed to translate these programs into a strong critical mass of researchers, especially young researchers able to contribute in all aspects of vaccine development, including basic research.
Governments and international agencies can speed HIV vaccine research by helping African national regulatory authorities (NRAs) improve their ability to assess the safety and efficacy of the products being tested and oversee vaccine clinical research. Taking full advantage of sponsor/PI interaction with NRAs and ethics committees is also key. Bringing NRAs and ethics committees together for joint reviews of multi-center or multi-country clinical trial applications (CTAs) with participation of other expert regulators and in the presence of the sponsors/manufacturer and clinical investigators could also expedite the process as well as build capacity (guidelines for joint reviews are currently being developed by WHO).Joint reviews were successfully implemented by the African Vaccine Regulatory Forum to fast track approval of ebola vaccine candidates [23]. As joint reviews are developed in Africa, country and regional level regulatory and legal frameworks should be established, similar to those used by international agencies that facilitate the protected sharing of proprietary information necessary for the review process. Presubmission meetings between NRAs and local investigators are mutually beneficial and confidence-building and will increase capacity to review and assess the products going into very early phases.
Advancing these recommendations depends on support and participation from African governments, researchers and advocates. The current reliance on funding from the North can have the unintended consequence of limiting the ability of African researchers to choose which studies they conduct, even though they give essential and valuable nonfinancial support. Increasing Africa’s financial commitment to vaccine research would more effectively drive the Pan-African HIV vaccine development agenda.
A sustainable network of African vaccine research stakeholders – to include researchers, communities, advocacy groups and other civil society partners –would facilitate greater African participation and advance many of the recommendations outlined here.
The former African AIDS Vaccines Program (AAVP) provides lessons on how best to establish and operate such a network. A virtual network may be more sustainable and would likely require less external funding than an organization with a legal entity, office and staffing. Built upon a broadly representative online survey of African researchers in addition to discussions such as those at the ICASA meeting in December 2015, key stakeholders outlined goals and developed an action plan to establish a clear vision for this African virtual network. To address the core needs of the African scientific community, this vision has taken shape with the continuing input of stakeholders through conference calls and subsequent meetings, most recently at the HIVR4P conference in Chicago in October 2016.
The newly formed “African AIDS Vaccine Virtual Network” or AAVVi. net will be a user-driven resource, with no legal entity, no staff, and no membership. Instead, AAVVi.net’s activities will be overseen and coordinated by a steering group and implemented by rotating working groups. An advisory group to AAVVi.net, the AAVVi.net Champions together with the Global HIV Vaccine Enterprise and with leadership from Pontiano Kaleebu, MRC/UVRI Uganda, is tasked to define governance and operational structures for this newly formed virtual network. AAVVi.net’s nimble structure will allow the network to react to the needs of the African HIV vaccine community in real time while strengthening communications and collaboration among each other, and with regulatory structures, government bodies and media; facilitate expanded training and capacity development opportunities; and advocate for greater African funding and leadership for HIV vaccine development and testing.
The need to develop an efficacious, safe and affordable HIV-1 vaccine remains a priority. With sub-Saharan Africa contributing the highest proportion of new infections, vaccine efforts have to ensure that Africa remains central to vaccine development and research and for this to be meaningful, we have provided a four pillar strategy to support development of effective HIV vaccines for Africa that address among others viral diversity, effective clinical trial designs, strengthening regulatory capacity and increasing participation through increased funding from Africa and networking. The newly created African AIDS Vaccine Virtual Network will provide new opportunities to facilitate this Pan-African HIV vaccine development agenda.
The authors have declared that no competing interests exist.
We thank the many stakeholders who helped shape the recommendations outlined in this commentary including the organizing committee and the participants of the Kigali meeting (see http://vaccineenterprise.org/ timely-topics/hiv-vaccine-development-pan-african-considerations for a list of advisors and participants). We also thank the Global HIV Vaccine Enterprise and the International AIDS Vaccine Initiative (IAVI) for sponsoring and the Rwandan Biomedical Research Center for co-hosting the meeting. Special thanks to Dr. Patricia Fast and Adam Winters for assisting with the preparation of this article and Dr. Morgane Rolland and Dr. Marcel Tongo for helping with the section on viral diversity
All authors were involved in the conception, and discussed the content and direction of this manuscript. AM drafted and revised the manuscript. GS, WS, SN, PK revised the manuscript providing additional critical intellectual input. All authors have read and approved the final manuscript.
Download Provisional PDF Here
Article Type: Review Article
Citation: Manrique A, Scarlatti G, Snow W, Nsanzimana S, Kaleebu P (2017) Toward a PanAfrican HIV Vaccine Development Agenda. J HIV AIDS 3(2): doi: http://dx.doi.org/10.16966/2380-5536.139
Copyright: © 2017 Manrique A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access