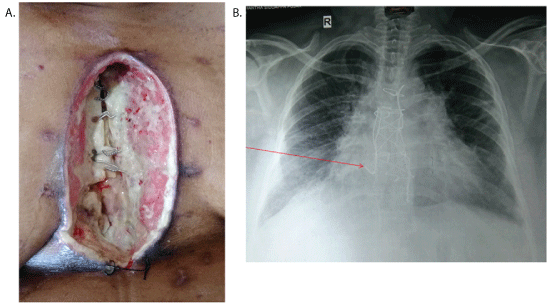
Figure 1: A) Sternal dehiscence.
B) Chest X-ray (Arrow shows sternal wire cut through).


Haranal Maruti Y1* Madhusudan G2
1Department of Cardio-Thoracic and Vascular Surgery, BGS Global Hospital, Bengaluru, Karnataka, India*Corresponding author: Haranal Maruti Y, Department of Cardio-Thoracic and Vascular Surgery, BGS Health and Education city, #67, Uttarahalli Road, Kengeri, Bangalore-560060, Karnataka, India, Tel: +91 9900972599; E-mail: marusurg@gmail.com
Sternal wound infection and dehiscence have been reported to occur in 0.2–10% of cases following the sternotomy with a mortality rate of 5 to 20%. The most devastating complication of sternal dehiscence is mediastinitis, which increases mortality to 50% hence, need a quick and aggressive management.
There are numerous treatment modalities available, ranging from multiple debridements to muscle/myocutaneous flaps. A muscle flap gives stable coverage, increased blood supply to the wound and protects the underlying vital structures. We report a case of sternal dehiscence following coronary artery bypass surgery treated successfully with bilateral pectoralis muscle flap (Double breasting).
Sternal dehiscence; Coronary artery bypass grafting (CABG); Mediastinitis; Myocutaneous flap
Sternal wound infection and dehiscence have been reported to occur in 0.2–10% of cases following sternotomy and mortality rates in such cases range from 5 to 20% [1]. The risk factors include diabetes, prolonged surgery time, prolonged postoperative ventilation, previous surgery, postoperative dialysis, postoperative haemorrhage, smoking, low cardiac output stage, use of either left internal mammary artery (LIMA) or right internal mammary artery (RIMA) or both and obesity.
The most devastating complication of sternal dehiscence is mediastinitis with an incidence of 47% and carries a mortality as high as 50% [2].
There are different classification systems described for sternal wound infections. Deep sternal wound infection (DSWI) was defined by the modified US Centers for Disease Control and Prevention (CDC) criteria for surgical site infection [3]. The diagnosis required at least 1 of the following criteria: 1) organisms cultured from the mediastinal tissue or fluid obtained during surgical operation or needle aspiration; 2) clinical evidence of mediastinitis seen during surgical operation; or 3) fever, chest pain, or sternal instability with either purulent discharge from the mediastinal area or positive culture of organisms from blood or mediastinal discharge. These definitions clarify the site of infection, but do not keep exact correlation with the existing real anatomical change.
The El Oakley criteria [4] were used to classify DSWI. This classification system divides DSWI into types I–IV, with type III and IV further subdivided into A and B according to time of onset after surgery, number of risk factors present, and previous treatment trials. A late onset of symptoms, multiple risk factors, and previous treatment trials would correspond to a higher classification.
Jones et al. [5], suggested for the first time a classification based on the affected anatomical site but still using as parameter the presence of infection. Greig et al. [6] classified sternal wound infections considering the regional location of the wound.
Currently, management of sternal wounds involves a multidisciplinary approach. Time-sensitive, nonsurgical management techniques include early debridement, microbiological analysis, and broad-spectrum antibiotics. A relevant development to sternal wound issues has been the development of vacuum-assisted closure devices (VAC) by Argenta and Morykwas [7] was, which serve as a bridge between debridement and reconstruction.
Managing infected sternal wounds changed with the introduction of principles of wide debridement and muscle/myocutaneous flap transposition. This management strategy of sternal wound infections was instituted in 1976 when Lee et al. [8] introduced the concept of flaps to reduce dead space in the anterior mediastinum by using the greater omental flap. In 1980, Jurkiewicz et al. [5] introduced the concept of muscle and myocutaneous flaps, which dramatically improved effectiveness of management of sternal dehiscence and infection. Mathes also contributed with the concept of using muscle flaps in wounds with osteomyelitis [9].
The advantages of muscle flaps are: a stable coverage, increased vascularity of the wound and also in case of breakdown of the wound prevents the exposure of vital structures. The selection depends on the presence of infection, tissue and skin loss, bony involvement and the mediastinitis.
In the clinical evaluation of suspected mediastinitis or sternal dehiscence, careful repeated examination of the patient is warranted. If the patient has multiple risk factors for dehiscence or impairment to wound healing, he or she must be examined at closer intervals. Findings of erythema, fever, tachycardia, increased leukocyte count, purulent discharge, and sternal instability are clinical indicators of sternal dehiscence.
If clinical deterioration of the patient or further signs of breakdown are observed (ie, increased erythema, drainage, separation of incision), immediately obtain wound cultures, administer broad-spectrum antibiotics, and perform swift aggressive debridement. Follow with either the vacuum-assisted closure (VAC) device (to serve as a bridge to reconstruction) or reconstruction with flap coverage or rigid sternal plating. This combination can reduce the incidence of mortality, decrease hospital stay, rapidly propel the patient’s recovery from thoracic surgery, and avert the severe complications of mediastinitis.
Fifty one years old female patient was referred to our hospital for coronary artery bypass grafting (CABG). She was short statured with BMI of 37.2. She had bilateral foot drop with paraparesis. She was diabetic and hypertensive. She was a case of chronic renal failure and had undergone three caesarean sections in the past. On examination hemodynamic parameters were normal. Serum creatinine was 1.9 mg/dl; serum cholesterol was 300 mg/dl and HbA1C of 8.4%.
Coronary Angiogram showed LAD- 80% stenosis, LCX-70% stenosis and RCA -100% occlusion with retrograde filling.
2D Echo revealed good left ventricular function (LV EF- 58%) without any regional wall motion abnormalities.
As the patient had bilateral foot drop with paraparesis, was obese and diabetic and had negative left Allen’s test with Doppler evidence of diffuse calcification the left radial artery (hence not used), we used bilateral skeletonized internal mammary arteries for Coronary artery bypass grafting (CABG).
The patient underwent off pump CABG – LIMA was sequenced to Diagonal and LAD, and in situ RIMA to distal RCA. There were no graftable Obtuse Marginals (OMs). The sternum was closed using Robicsek technique.
Perioperative period was uneventful and the patient was discharged on 5th postoperative day.
After two weeks, she was presented with sternal dehiscence (Type 3a – Jone classification). She was afebrile with stable hemodynamics. The discharge from the wound was sent for culture and sensitivity, and started on intravenous broad-spectrum antibiotic. Wound was debrided and Vacuum Assist Closure (VAC) device was used with a hope of secondary closure once the healthy granulation tissue appears. Culture revealed scanty growth of Klebsiella pnemoniae sensitive to meropenem, occasional pus cells. The patient was started on IV Meropenem. However, there was no adequate response to VAC dressing after two weeks. Instead sternum became osteomyelitic and sternal wires were cut through (Figure 1A and 1B) and the heart was exposed. There was a concern about bony chips and the sternal wires injuring the heart. Moreover, the patient had excruciating pain, hence the plastic surgeon was consulted. In view of patient’s condition, thorough debridement and an emergency muscle flap coverage of the defect was planned despite unavailability of culture report.

Figure 1: A) Sternal dehiscence.
B) Chest X-ray (Arrow shows sternal wire cut through).
Under general anaesthesia sternal wires were removed. Right half of the sternum with few ribs was removed till the costo-chondral junction. Wound was debrided thoroughly to the point of fresh bleeding. Bilateral pectoralis muscle flaps were raised on thoracoacromial pedicle and released from their insertion and rotated medially to cover the defect (double breasting) (Figure 2A and 2B). Double breasting also covered a part of the lower defect. Tension free secure wound closure was done in three layers with suction drains placed one above and one below the flap, one in each mammary space. Lower residual defect was closed using subcutaneous fat and the skin.
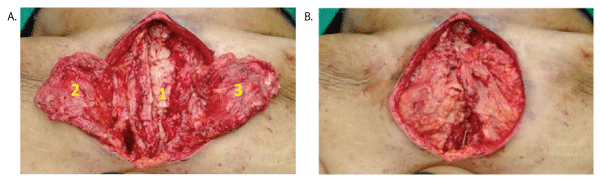
Figure 2: Intraoperative pictures- A) 1- Debrided wound; 2-Left pectoralis flap; 3- Right pectoralis flap; B) Double breasting of pectoralis flaps.
The culture report showed multidrug resistant Klebsiella pnemoniae, which was sensitive only to colistin and tigecyclin. She was started on Colistin continued for 14 days along with meropenem as per her creatinine clearance.
The perioperative period was uneventful. She was discharged after two weeks and skin sutures were removed after three weeks. Patient is under follow up and doing well.
The development of devastating sternal wound infections after sternotomy for CABGs and other major cardiac procedures has been a major concern for surgeons since the development of the median sternotomy. The prompt and quick attention to these infections is must to avoid the development of osteomyelitis, mediastinitis and systemic sepsis.
The very fact that there are various options described for the management of sternal dehiscence makes it obvious that the ideal option still eludes us. A combination of flaps may be sometimes required, particularly to deal with difficult areas such as the inferior third of the sternum.
Use of omentum involves laparotomy and opening of new cavity adjacent to area of infection. Disadvantages have been reported with titanium plate fixation. Some studies have reported a high incidence of plate removal (8% to 25%). This is explained by the fact that sternocostal junctions are highly mobile articulations during respiration and this motion is more active in more far location from the sternum. The size mismatch between the titanium plate and the thoracic wall requires a long plate, and it may result in chest wall pain, which is the main cause of titanium plate removal. Titanium plate fixation has potential drawbacks, including bleeding and lung injury. The cost problem is another disadvantage to the use of titanium plate fixation.
The principles of reconstruction involve radical debridement, tension free flap coverage so as to obliterate the dead space. There are various muscle or myocutaneous flaps available in reconstructive armentarium. They can be used as single or in combination based on the defect to be covered.
Debridement and immediate closure with muscle or myocutaneous flap has gained popularity since 1980s.
Pectoralis muscle has stood the test of time in the coverage of these defects. It is a type V Mathes-Nahai classification flap, it has 2 dominant vascular contributions. Thus, it is unaffected by the harvesting of the internal mammary arteries (IMA) for coronary bypass. The pectoralis flap can be transposed into the mediastinum based on the more commonly used thoracoacromial pedicle or as a turnover flap based on internal mammary perforators. This muscle usually is the first choice in flap selection because of its proximity and relative ease of harvest. It is presently the favoured muscle flap for covering infected sternotomy wounds.
A major limitation of pectoralis major flap has been the coverage of the inferior third of the sternotomy wound. Studies have shown that the lower portion of wounds, near the xiphisternum, is the most common site of dehiscence after flap repair [10]. Tripedicle pectoralis major myocutaneous flap has been used by some authors to address this problem [11].
The advancement of the pectoralis major bilaterally is efficient in obliterating the dead space and covering the entire defect with tension-free vascular tissue. Advantages of double breasting include: this obliterates the cavity and prevents hematoma/seroma formation, provides some stability to the sternum and prevents the paradoxical movement of the sternum, by using the double breasting technique, overlapping suture lines are avoided ( if there is dehiscence of one suture line, the other suture line acts as a protective barrier and prevents complete disruption), it can be used in patients who have undergone CABG using both internal thoracic arteries [12,13].
A study by Vijay Yashpal Bhatia et al. [14] showed that the Pectoralis major flap is an effective method in the reconstruction of the Sternal defect caused following CABG. It not only provides sufficient volume to fill the entire mediastinum but also affords resolution of the infected wound with favourable outcomes.
Similarly, Parag Sahasrabudhe et al. [15] showed that the double breasting technique of the pectoralis major muscle flaps with rectus sheath extension is efficient in covering the entire length of the defect and can reduce the morbidity, without affecting the function of the shoulder joint.
In our case, the patient was diabetic, hypertensive, had chronic renal disease, morbidly obese developed sternal dehiscence with mediastinitis following CABG (Bilateral internal mammary arteries). The patient was treated successfully with radical debridement and double breasting of pectoralis flaps covering the upper and middle thirds of the sternum. Lower third of the defect was covered with three layer closure of subcutaneous tissue and skin. We couldn’t use omentum or recuts abdominis flap/ Latissimus Dorsi (LD) flap to cover the lower defect because of her obesity and diabetes. The patient made a great recovery and doing well.
The authors declare that they have no conflict of interest.
Download Provisional PDF Here
Article Type: Case Report
Citation: Maruti HY, Madhusudan G (2017) Double Breasting for Sternal Wound Dehiscence following CABG. J Hear Health 3(3): doi http://dx.doi.org/10.16966/2379-769X.141
Copyright: © 2017 Maruti HY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access