Introduction
Despite a global decrease in incidence, gastric cancer is the fourth most common malignancy and the second leading cause of cancer death in both sexes worldwide [1,2]. In the western world the incidence of adenocarcinomas of the esophagus and gastroesophageal junction (GEJ) is rising. Although the clinical outcome of adenocarcinomas in esophagus, stomach and GEJ has gradually improved, patients still have a poor survival, with an overall 5-year survival of 20% [3]. Therefore a better understanding of molecular aberrations associated with gastric cancer is needed in order to identify new diagnostic and therapeutic strategies.
Non-coding RNAs (ncRNAs) make up a major fraction of human RNA and modulate gene expression [4,5]. The micro RNAs (miRNA), a class of 21-25 nucleotide ncRNAs, have received increasing attention in the last decade, due to their role in the pathogenesis of many human malignancies [6]. Recently, also the long (>100 nucleotides) ncRNAs (lncRNAs) have been identified as regulators of gene transcription [4,5]. One of the lncRNAs is the HOX transcript antisense intergenic RNA (HOTAIR) which is transcribed from the HOXC locus [7]. HOTAIR binds to the polycomb-repressive complex 2 (PRC2) and targets it to the HOXD locus where it inhibits HOXD gene expression. The PRC2 is a histone H3 lysine 27 (H3K27) methylase involved in developmental gene silencing and cancer progression [8,9]. Over-expression of HOTAIR in cancer cells seems to reprogram the polycomb binding profile from that of an epithelial cell to that of a mesenchymal embryonic fibroblast, and this is associated with a high risk of metastatic progression [10].
The development of gastric cancer is associated with aberrant gene expression of HOX genes and miRNAs encoded within the HOX cluster [11-13], which is in part due to epigenetic changes [14,15]. Overall this suggests that there is a general change in gene expression from the HOX cluster during gastric oncogenesis. We therefore wanted to examine the expression and prognostic value of HOTAIR, a lncRNA encoded within the HOX cluster, in gastric cancer biopsies as well as its regulation.
Materials and Methods
Patient selection and collection of biopsies
This cohort study includes patients operated from July 2008 to November 2009 for adenocarcinoma of the stomach, esophagus or GEJ. All individuals provided an informed written consent. Collection of biopsies was approved by the Danish ethical committee (journal number H-B-2008-049) and the establishment of a biobank was approved by the Danish Data Protection Agency (journal number 2008-41-2138). Biopsies were taken from both tumor tissue and the nearby adjacent normal tissue. Tumor content was evaluated in biopsies taken adjacent to those used for RNA extraction and ranged between 35 and 70%. Pre-defined clinical and paraclinical data were extracted from medical charts and pathology reports.
Cell cultures
Human SNU638 gastric adenocarcinoma cell cultures were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) while the normal gastric epithelium cell line HFE145 (kindly provided by Professor Hassan Ashktorab, Howard University, Washington, D.C.) was maintained in DMEM medium (Invitrogen). Both media were supplemented with 10% fetal bovine serum (FBS) (Invitrogen), 100 µg/ml streptomycin and 100 U/ml penicillin (Invitrogen) at 37ºC, and 5% CO2 humidified atmosphere. HOTAIR expression was 1000 fold higher in SNU638 cells compared to the HFE145 cells (Figure S2), therefore over-expression studies were performed in HFE145 cells while knock down experiments were carried out in the SNU638 cells.
In vitro drug treatment
One day before stimulation, 500.000 SNU638 cells were seeded in 6 well-plates. The SNU638 cells were treated with 100 µM 5-FU (Hospira Nordic AB, Stockholm, Sweden), 10 µM Nutlin-3 (Sigma-Aldrich, St. Louis, MO) or with 2.5 µm, 5 µM, or 10 µM Vorinostat (Patheon Inc., Mississauga, Ontario, Canada) for 24 hrs prior to RNA extraction. Nutlin-3 and Vorinostat were dissolved in Dimethyl sulfoxide (DMSO). The final % DMSO for the different treatments were 0.0025% for treatment with 2.5 µM Vorinostat, 0.005% for treatment with 5 µM Vorinostat, and 0.01% for treatment with 10 µM Vorinostat or Nutlin3. Negative control cells were treated with 0.01% DMSO. All treatments were done in triplicates.
Transfection
Prior to the transfection, 500.000 SNU638 cells were seeded in 6 well-plates for 24 hrs. The SNU638 cells were transfected using 5 µl TurboFectTM in vitro Transfection Reagent (Fermentas, Burlington, Canada), and mixed with 50 nM TaqMan® MicroRNA Assay hsa-miR-449 (Applied Biosystems, Foster City, CA) or 50 nM siRNA FexiTube Gene Solution short interfering histone deacetylase 1 (siHDAC1), siHDAC2, or in combination (Qiagen, Hilden, Germany). 300 µl GIBCOTM Opti-MEM I (Invitrogen, Carlsbad, CA) was added and the mixture was incubated for 20 min before being applied to the cells. RNA and protein was extracted 24 hrs post transfection. Negative control cells were treated with a scrambled siGLO siRNA (Thermo Scientific, Waltham, MA). All transfections were done in triplicates.
Measurement of cell death
To make sure that the results presented in this paper represented gene expression from living cells, we tested the effect of miR-449, HDAC siRNAs, and drug treatment on SNU638 cell viability by counting the number of cells free flowing in the culture media 24 hrs after transfections and drug treatments. Cells were counted using the Countess automated cell counter (Invitrogen). Negative controls were incubated in pure culture media or media containing 0.01% DMSO. All experiments were done in quadruplicates. As expected, siHDAC and Vorinostat treatment induced cell death to some degree as has been shown for other gastric cancer cell lines [16,17]. Also the DNA damaging drugs 5-FU and Nutlin-3 induced cell death (Figure S3 A and B). However, most cells survived the treatments and the surviving cells looked healthy allowing the measurements presented in this paper.
Cell proliferation and migration
The role of HOTAIR on cell proliferation and migration in gastric cancer cells was investigated by transfecting HFE145 cells with the LZRSHOTAIR expression vector (Addgene plasmid 26110) [10]. 400.000 HFE145 cells were seeded in 10 cm plates 24 hrs prior to transfection with 4 µg LZRS-HOTAIR or 4 µg empty LZRS vector using TurbofectTM (Fermentas) as described above. 24 hrs after transfection 10.000 cells/ well were seeded in a CIM-plate 16 for proliferation and 20.000 cells/well were seeded in the upper chamber of E-plate 16 for migration studies (ACEA Bioscience, San Diego, CA/Roche Diagnostics GmbH, Manheim, Germany). Proliferation and migration was followed real-time over 36 hrs and 12 hrs respectively, with xCELLigence impedance analysis using the RTCA DP instrument (ACEA/Roche). This method allows continuous measurement of cell proliferation or migration by measuring the electrical impedance over gold electrodes incorporated on the well bottom for proliferation measurement and incorporated on the underside of a microporous polyethylene terephthalate dividing an upper and lower chamber for migration measurement. N=6 for proliferation and N=4 for migration studies. Calculations were performed using the RTCA software (ACEA/Roche).
RNA extraction and qPCR
Biopsies from the gastric tumors or the normal adjacent tissue were collected during surgery and placed in RNA later (Applied Biosystems) immediately after collection. Prior to storage at -80ºC the RNA later was removed and 1 ml Trizol was added (Invitrogen). The tissue samples were homogenized on a Tissuelyser (Qiagen) and RNA from tissue and cell culture was isolated according to the manufactures protocol for Trizol RNA isolation (Invitrogen). RNA concentrations were measured using a NanoDrop ND-1000 Spectrophotometer. RNA quality was tested on a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California) according to the manufactures protocol and all RNA samples had a RNA integrity number (RIN) value of ≥8. cDNA was synthesized from 1 µg isolated RNA using Super Script III First-Strand Synthesis SuperMix for qPCR (Invitrogen). Gene expression was analyzed by quantitative reverse transcription PCR (qRT-PCR) using SYBR GreenER q-PCR SuperMix for ABI PRISM (Invitrogen). The reactions were run on an ABI PRISM 7900HT Sequence Detection System (SDS) from Applied Biosystems. mRNA expression was normalized to GAPDH and expression was calculated as the difference in expression level of tumors or treated cell lines compared to normal tissue or control cell lines. Efficiency of primers was estimated by studying the slope of the standard curve. All standard curves had a slope between -3.3 and -3.6 indicating a primer efficiency of 90-100%. mRNA primers were designed using Primer-Blast software from NCBI [18], to span at least one intron in order to ensure amplification of cDNA only (for mRNA primers see Table S1).
Western blotting
Proteins were purified using the RNA/DNA/Protein Purification Kit (Norgen Biotek Corpoation, Throld, Canada). 400 µg protein was loaded pr. well and separated on polyacrylamide gels (NuPAGE® Novex® 4-12% BisTris Gel 1.0 mm, Invitrogen). Proteins were blotted onto a polyvinylidene difluoride membrane (Invitrogen) and incubated over night with primary antibodies against HDAC1 (sc-81598), HDAC2 (sc-7899), p53 (sc-126), Calnexin (sc-11397) from Santa Cruz Biotechnology, Dallas, Texas. Proteins were visualized by chemiluminiscence (AmershamTM ECLTM Select Western Blotting Detection Reagent, GE Healthcare, Little Chalfont, UK) using LAS-1000 Pro v. 2.6 (FujiFilm, Tokyo, Japan).
Definition of cut-off value
HOTAIR expression was transformed into a binary variable by dividing the cohort into a low and high expression group. We used the same approach as Wu et al. [19] who separated the tumours into two groups: Low HOTAIR expression = the expression seen in the two-thirds of the tumors with the lowest expression. High HOTAIR expression = the expression seen in the last third of the tumours with the highest expression. Gupta et al. [10] suggested a cut-off of 125 fold increase in HOTAIR RNA expression for the separation into high and low HOTAIR groups, and in this study the cut-off separating the high and low expression group was 93 fold change.
Statistical evaluation
Overall survival was defined as the time from the date of surgery to death. Time-to-event data were estimated by the Kaplan-Meier method and compared using the log-rank test and univariate regression analysis. A multiple Cox proportional hazard regression model was used to identify independent prognostic factors with a 95% CI. As recommended [20], only four variables were included in the analysis due to the low number of events. The most significant variables in the univariate analysis were selected. The statistical analyses were performed by use of SPSS version 19 and Graph Pad Prism. qRT-PCR results were expressed as mean values of at least three independent experiments measured in three technical replicates, and analyzed by unpaired two-tailed t-test. The differences between data sets were considered significant at p values ≤0.05. Error bars = ±SEM unless otherwise indicated.
Results
Patients
40 patients were enrolled out of a total of 111 patients operated from July 2008 through November 2009 at the Department of Gastroenterological Surgery at the university hospital of Copenhagen. All patients included in the study were native Danish Caucasians. 10 patients received chemotherapy, all in an adjuvant setting. 7 patients received postoperative adjuvant treatment consisting of 5 cycles of fluorouracil (5- FU) plus leucovorin and locoregional radiotherapy (1, 8 Gy x 25 fractions, 5 fractions/week). Additionally, 3 patients received 6 cycles of taxotere, carboplatin, and capecitabine.
Survival of study patients was not statistically different from those who underwent surgery without collection of tumor tissue in the study period (median survival 32.2 months (26.4-31.3) vs. 26.9 (22.4-31.3). HR=1.65 (0.9-2.8) p=0.078) (Figure S1). No significant differences in patient outcome between stomach and GEJ/E/G cancers were observed.
Of the 40 patients included, 3 patients were lost to follow-up and in 4 cases the quality of the tissue samples was too poor. The patient demographics and clinico-pathological characteristics are shown in Table 1. For additional patient characteristics see supplementary Table S2.19 patients (58 %) had died at the time of analysis. The median followup time was 22 months (range 1-37 months).
| Demographics |
| Variable |
Number of patients |
% |
| Gender |
Male |
25 |
76 |
| Female |
8 |
24 |
| Performance status |
0 |
14 |
42 |
| 1 |
19 |
58 |
| Age |
Median |
63 years |
(36-90) |
| Distant metastasis |
Yes |
3 |
9 |
| No/Unknown |
30 |
91 |
| Clinico-pathological Characteristics |
| Variable |
Number of patients |
% |
Lauren
Classification |
Diffuse |
12 |
36 |
| Intestinal |
21 |
67 |
| Localization |
Esophagus/GEJ |
19 |
58 |
| Stomach |
14 |
42 |
| Nodes with carcinoma |
N 0-1 |
17 |
52 |
| N 2-3 |
16 |
48 |
Table 1: Patient demographics and Clinico-pathological Characteristics.
Over-expression of HOTAIR was an independent predictor of death
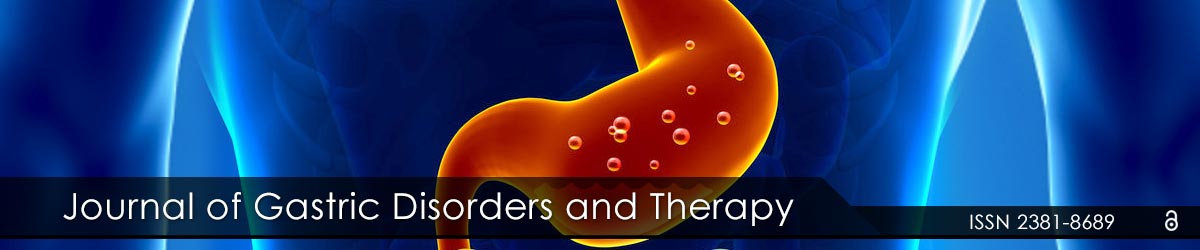
We found that the expression of HOTAIR in tumors compared to adjacent normal tissue ranged from unchanged, to a nearly 700-fold increase (Figure 1A). Those in the HOTAIR high expression group had an upregulated HOTAIR expression ranging from 93 to nearly 700 fold in the adenocarcinomas compared to normal gastric epithelia (Figure 1A). Patients with low HOTAIR expression survived significantly longer with a median overall survival of 22.9 months compared to the high HOTAIR group with a median survival of 16.7 months (p=0.026) (Figure 1B).
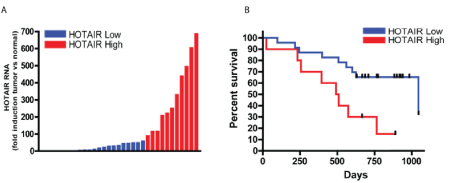
Figure 1: High expression of HOTAIR was associated with poor survival: For each patient, the mRNA expression level in tumor tissue was compared to that of the corresponding normal tissue.
(A) qRT-PCR analysis of HOTAIR in 33 gastric cancer tumors. Patients with the top 33% of HOTAIR fold increase were categorized as HOTAIR high and the remaining two thirds were defined as HOTAIR low.
(B) Kaplan-Meier curve for overall survival of the 11 HOTAIR high (red) and 22 HOTAIR low (blue) patients shown in A (p=0.026).
Examining the different prognostic factors on survival we found that age >=65 years p=0.032 (HR=3.2 (95% CI 1.1-9.3)), TNM stage III+IV p=0.044 (HR=3.7 (95%CI 1.4- 10)) and high HOTAIR expression p=0.026 (HR=3.3 (95% CI: 1.2-9.6)) was identified as the only significant predictors of death in the patient population (Table 2).
| Group |
Log-rank (Mantel-Cox) test |
Chi square |
p value |
Survival curves
significantly different? |
Hazard Ratio (Mantel-Haenszel) |
95% CI of ratio |
| Sex |
Male vs Female |
1,19 |
0,275 |
No |
1,8 |
0,63 to 5,1 |
| Age |
>=65 vs <65 |
4,61 |
0,032 |
Yes |
3,2 |
1,1 to 9,3 |
| Localisation |
GEJ vs Fundus/Corpus/Antrum |
0,37 |
0,545 |
No |
1,3 |
0,52 to 3,5 |
| |
GEJ vs Corpus/Fundus |
0,03 |
0,864 |
No |
0,89 |
0,23 to 3,4 |
| |
GEJ vs Antrum |
0,86 |
0,353 |
No |
1,7 |
0,56 to 5,0 |
| |
Corpus/Fundus vs Antrum |
0,66 |
0,418 |
No |
2,0 |
0,38 to 10 |
| Lauren Classification |
Diffuse vs Intestinal |
0,16 |
0,686 |
No |
1,2 |
0,46 to 3,2 |
| Perineural Invasion |
Yes vs No |
1,07 |
0,301 |
No |
1,7 |
0,61 to 4,7 |
| Intra vascular growth |
Yes vs No |
0,27 |
0,602 |
No |
1,6 |
0,28 to 9,0 |
| TNM |
I+II vs III+IV |
4,00 |
0,044 |
Yes |
3,7 |
1,4 to 10 |
| |
T3/4 vs T1/2 |
0,46 |
0,498 |
No |
1,4 |
0,51 to 4,1 |
| T |
T3 vs T1/2 |
0,01 |
0,942 |
No |
0,95 |
0,21 to 4,2 |
| |
T4 vs T1/2 |
1,00 |
0,318 |
No |
1,8 |
0,58 to 5,3 |
| |
T4 vs T3 |
1,21 |
0,272 |
No |
1,9 |
0,60 to 6,1 |
| N |
N0 vs N1+ |
0,03 |
0,868 |
No |
1,1 |
0,41 to 2,9 |
| M |
M0 vs M1 |
0,23 |
0,629 |
No |
0,67 |
0,13 to 3,5 |
| HOTAIR |
High vs Low |
4,94 |
0,026 |
Yes |
3,334 |
1,2 to 9,6 |
Table 2: Survival analysis.
Over-expression of HOTAIR induced cell proliferation of HFE145 cells
To examine the role of HOTAIR on cell proliferation and migration we over-expressed HOTAIR in the normal gastric epithelial HFE145 cells. We found that HOTAIR transfection halved the doubling time from 20.9 hrs (±1.6 hrs) to 10.8 hrs (±0.5 hrs) (p<0.05) (Figure 2 A and B). Thus, high expression of HOTAIR doubled cell proliferation thereby increasing oncogenic potential of the cells. Over-expression of HOTAIR did not affect migration over a membrane of the HFE145 cells during a 12-hour period (Figure 2 C and D), meaning that we did not find an increased metastatic potential in these experiments.
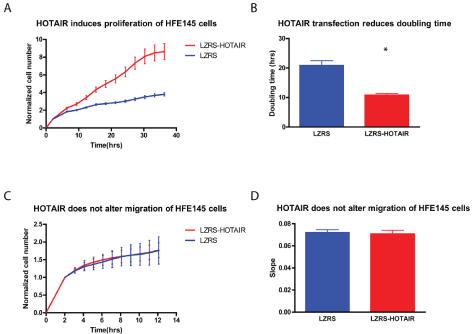
Figure 2: HOTAIR induced proliferation of gastric HFE145 cells: HFE145 cells were transfected with either the LZRS-HOTAIR expression vector (red) or the empty LZRS control vector (blue).
(A, B) HOTAIR induced proliferation, reducing HFE145 doubling time by 50% (p<0.05).
(C, D) In contrast HOTAIR did not promote migration of the cells. N=6 for the proliferation assays and N=4 for the migration assays.
* Denotes significantly different from control cells p<0.05.
Inhibition of HDAC suppressed HOTAIR expression
We have previously shown that miR-449 expression is down regulated in gastric cancer [21]. We have further more shown that miR-449 targets the HDACs and inhibits growth of gastric tumor cells [21]. The hox cluster in part operates under epigenetic control, hence miR-449 could regulate the expression. Furthermore Chiyomaru, et al. [22] has shown that HOTAIR is a direct target of miR-34a, which is a miRNA with an identical seed sequence to miR-449. Identical seed sequences give miRNAs the ability to target the same sequences, suggesting a role for miR-449 in HOTAIR down regulation. We therefore hypothesized that miR-449 would inhibit cell growth in gastric tumor cells by directly or indirectly regulating HOTAIR, and therefore examined how miR-449 over-expression affected HOTAIR expression. We found that transfection of miR-449 into SNU638 cells led to a reduction in HOTAIR expression (Figure 3A). miR-449 target multiple signaling pathways and over-expression of this miR was found to activate p53 (Figure 3B) [21]. Activation of p53 either by 5-FU or by Nutlin-3 treatment increased HOTAIR expression (Figure 3 C and D) and thus could not explain the reduction in HOTAIR expression following miR-449 transfection. We then searched for other mechanisms by which miR-449 could regulate HOTAIR expression, and found that miR-449 targeted HDAC1 and -2 (Figure 4 A and B). Therefore, we tested how siRNA knockdown of HDAC1 and -2 affected HOTAIR expression. First we confirmed the knockdown of HDAC1 and -2 by RTq-PCR and Western blotting (Figure 4 C and D). We found that siRNA knockdown of HDAC1 and -2 alone and especially combined led to a marked reduction in HOTAIR expression (Figure 4E). The effect of HDAC inhibition on HOTAIR expression was further confirmed with a dose dependent reduction in HOTAIR expression by the HDAC inhibitor Vorinostat (Figure 4F). Thus, we conclude that miR-449 regulates HOTAIR expression by either directly targeting HOTAIR or indirectly through the inhibition of HDACs.
Figure 3: HOTAIR expression was reduced by over-expression of miR-449 but was not reduced by p53 activation
(A) HOTAIR expression in SNU638 cells was reduced following transfection with miR-449.
(B) miR-449 transfection increased p53 expression as demonstrated by Western blotting.
(C, D) HOTAIR expression determined by qRT-PCR was increased following p53 activation induced by 5-FU or Nutlin-3 of SNU638 cells. All treatments were 24 hours.
* Denotes significantly different from control cells p<0.05.
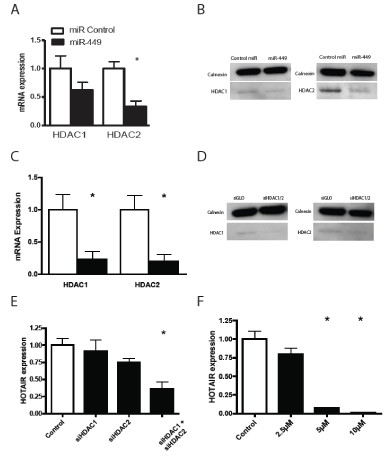
Figure 4: Inhibition of HDACs lead to a reduced HOTAIR expression in SNU638 cells.
(A, B) The expression of HDAC1 and HDAC2 mRNA and protein was reduced following miR-449 over-expression. (C, D) RTq-PCR and Western blotting confirmed knockdown of HDAC1 and HDAC2. (E) HOTAIR mRNA expression was reduced following siRNA knock down of HDAC1 and HDAC2. (F) The HDAC inhibitor Vorinostat dose dependently reduced HOTAIR expression. All treatments were 24 hours.
* Denotes significantly different from control cells p<0.05.
Discussion
Local invasion and distant micro-metastases that are often not detectable at the time of surgery, are the main causes of cancer related deaths. Therefore, in order to individualize and intensify perioperative treatment, it is important to find biomarkers to identify patients at high risk of recurrence and short survival as early as possible, ideally at the time of diagnosis.
In this study we found, that HOTAIR is upregulated to a maximum of 690-fold in biopsies from esophageal, GEJ and gastric adenocarcinomas, and that high expression of HOTAIR correlated with poor survival. Induction of HOTAIR has also been found to be a powerful predictor of the development of distant metastasis and death independently of known clinical and pathological risk factors such as tumor size, stage and hormone receptor status in breast cancer [10], liver cancer [23], colorectal cancer [24] and recently in gastric cancer supporting the results presented here [25,26]. HOTAIR is expressed in the HOXC locus and represses the HOXD cluster by recruiting PRC2 [7]. Over-expression of HOTAIR is accompanied by an altered chromatin state, allowing PRC2 to be recruited to genes that normally do not bind to PRC2 in epithelial cells, causing a down regulation of the expression of multiple genes that suppress tumor metastasis [27]. Genes regulated by excessive HOTAIR expression include CDH1 target genes [24], which is associated with increased invasiveness and development of metastasis in gastric adenocarcinomas [28]. This could very well be one of the mechanisms by which HOTAIR overexpression predisposes to poor prognosis in gastric, esophageal and GEJ adenocarcinomas.
We have found that aberrant expression of HOTAIR encoded from the HOX cluster was associated with gastric cancer progression. In this study we demonstrated that HOTAIR over-expression induced cell proliferation of normal gastric epithelial cells paralleling observations from esophageal squamous cell carcinoma [29], gastrointestinal stromal tumors [30], colorectal [24], pancreatic [31], hepatocellular [23] and breast [10] cancers and cell lines. Together with earlier reports [10,13,24-26] it suggests that dysregulation of the transcriptional control of transcripts from the HOX cluster is important for gastric carcinogenesis. As shown in several studies, high HOTAIR expression causes an increase indistant metastasis of several cancer types including gastric cancer. We measured the noncancerous cell line HFE145 ability to migrate over a membrane after over expression of HOTAIR and did not find any increase in migration over a 12-hour period. We did not, however, investigate the effect of HOTAIR over expression on the cells’ ability to spread and metastasize by other means than migration, such as loss of adherence, expression of growth factors, and remodeling of chromatin state. Little is at present known about the processes that control HOTAIR expression. The precise transcriptional control therefore needs further elucidation. In this study, we showed that miR-449 caused a down regulation of HOTAIR, possibly by targeting HOTAIR directly or by targeting HDACs. Showing HOTAIR as a direct target of miR-449 is beyond the scope of this paper. HDACs deacetylate histones and tighten their interaction with DNA, resulting in the inhibition of gene transcription [32]. Here we showed that inhibition of HDAC1 and -2 leads to a loss of HOTAIR expression. Thus HDACs may inhibit the transcription of HOTAIR repressors, although this needs further investigation. The reduction of HOTAIR expression by siHDAC and vorinostat implies that treatment with HDAC inhibitors may have a role in gastric cancer therapy, which has also been suggested by Claerhout et al. [17]. In support of this, high HDAC expression is associated with poor prognosis in gastric cancer [16].
Conclusions
In conclusion, elevated expression of HOTAIR increased proliferation of gastric cancer cells and was associated with a poor prognosis in gastric cancer patients corroborating recent findings [26]. Our data show that miR-449 and HDAC may contribute to the regulation of HOTAIR in gastric cancer, however this needs to be investigated further. High HOTAIR expression in gastrointestinal tumors should be investigated further to stratify for increased cancer risk and possible need for more extensive surgery and/or aggressive perioperative chemotherapy.
Acknowledgments
Mette Hedegaard Moldaschl is thanked for expert technical assistance. This work was supported by Danish MRC (LFH), Novo Nordic Foundation (LFH), University of Copenhagen, Faculty of Health and Medical Sciences (JABS), Oncological Research Foundation (MG), and Roche (MG).
Author Contributions
JS, MG, LiB and BF acquired the data presented in this article. JS, MG and LFH obtained funding, designed the study, analyzed and interpreted data, and drafted the manuscript. JS, LiB, BF and LFH performed critical revision of the manuscript for important intellectual content. JS carried out the molecular biology and MG carried out the statistical analysis. BF performed the pathology assessment. LFH supervised the study. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.