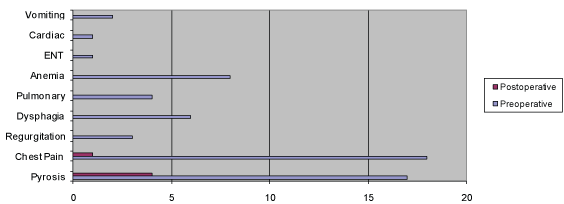
Figure 1: Incidence of presenting symptoms.

Eldo E Frezza1* Edoardo GP Frezza2
1Valley Baptist Medical Center, University of Texas, Texas, USA*Corresponding author: Frezza EE, East New Mexico University, Roswel lNM, 2101 Pease Street, Harlingen, Texas 78550, USA, E-mail: eefrezza@msn.com
While the prevalence of PEH in the adult population is about 5%, the literature shows that this prevalence increases 3 folds with age, particularly after age 40. The goals of this study are: 1) review what we know about the surgical treatment of Parahesophageal hernia and 2) given that this hernia is more prevalent in the geriatric population and that this population is more prone to complication, we will also focus on what can be eliminated from the surgery to make it smoother without compromising the success rate reporting our experience. We collected our data on elderly adults that underwent LPHR.
Study design: This is a retrospective study of patients undergoing laparoscopic paraesophageal hernia repair from June 2009 to December 2013 at our hospital. All patients stayed for a minimum of 1 night in the hospital and on postoperative day number 1 they underwent an upper GI swallow to rule out a intraoperative injury or post-operative leak. After the upper GI series, the patient was started on clear liquid diet, advanced to a full liquid as tolerated and sent home. Post-operative follow-up was scheduled at 2 weeks, 6 weeks, 3 months, 6 months, 12 months, 24 and 48 months after surgery. We also analyzed the literature to review what is current in the PHE treatment.
Results: We considered 32 patients; 10 males and 22 were females. Median age was 74 years with a range of 60 to 89. All patients had a preoperative barium esophagogram and were classified by hernia type. Patient population consisted of 14 type 2 hernias, 12 type 3 hernias, and 6 type 4 hernia. All the patients had esophagogastroduodenoscopy before surgery. Esophagitis was present in 29/32 patients. One patient mucosal changes on EGD. Preoperative lactate level and WBC were considered pre and postoperative, the ASA score by American Society of Anesthesiologists was 4 in 11 patients and 3 in the remaining. None of the patients were treated emergently as defined within the first 12 hours of presentation to the Emergency Department. Five patients were treated within 24 hours to admission of the emergency room and three patients within 48 hours of the admission. All the other patients were treated as an elective surgery. Twenty-eight patients underwent laparoscopic surgery with primary closure, 4 were closed with the BIOMESH as described in the method.
Conclusion: PHE is a disease more prevalent in the geriatric population. Given this we need to operated careful and eliminate all the steps which are not needed. In our study and literature review, we conclude that: 1) Mesh should be used only when the crura cannot be closed primarily with stitches such as large defect till we all agree on the best mesh to use absorbable or not; 2) gastrostomy tube or always a 2 full thickness gastropexy stitches to reduce the risk of PEH recurrence and gastric volvulus it is the most important manoeuvre if we think exclusively at the phatophysiology; 3) effort should be made to safely lengthen the esophagus off of underlying adhesions for proper GE junction placement. We suggest placing and endoscope to guide the esophageal dissection; 4) adding the antireflux procedure is not always necessary.
Paraesophageal hiatal hernia (PEH), consists of 5% to 10% of all hiatal hernias that present in the outpatient clinic for surgical repair [1]. 90% of paraesophageal hernia cases present as a type 3 hernia, meaning that 50% of the stomach is herniated into the mediastinum. Additional etiology includes type 2 and type 4 paraesophageal hernias. Type 2 is characterized by herniation of the gastric fundus into the chest, while type 4 represents herniation of the stomach as well as other viscera into the chest. Type 4 is considered the least prevalent subtype. Since PEH may present as an emergent condition, the majority of the current literature shows that the treatment of PEH relies on avoiding potential inherent complications to the stomach, such as strangulation [2-4]. However, because the natural history of the PEH shows that these hernias may not become clinically symptomatic, other schools of thought advocate for conservative management of these patients [4-6].
According to Stylopoulos and Rattner [6], only 20% of patients that present with paraesophageal hernias require repair. The lifetime complications of PEH include obstruction, acute dilatation, perforation, and bleeding of the stomach mucosa [7,8]. The current literature is inconclusive regarding the advantages of surgical repair over conservative observation of the PEH patient, but current clinical practice favors surgical repair [2,9-12]. The principles of surgical treatment are consistent in both open and laparoscopic PEH repair and include the following: excision of the peritoneal sac, reduction of the herniated stomach, dissection of the esophagus with caudal repositioning into the abdominal cavity, and repair of the diaphragmatic hiatus [13-15]. In order to avoid the potential return of the stomach into the chest, the surgeon may place a gastrostomy tube or perform gastropexy of the stomach to the abdominal wall.
The first laparoscopic approach for paraesophageal hernia was reported by Cuschieri in 1992 [16]. Although proven to be both safe and efficacious by multiple authors [17-21], controversy remains regarding open versus laparoscopic approach. Since the introduction of laparoscopy, the laparoscopic repair of paraesophageal hernia, (LPHR), has replaced the open repair in almost every institution, thereby increasing surgical repair of PEH overall. Given the minimal incision of the laparoscopic procedure, the short-term outcomes of LPHR are superior to open repair due to decreased chances of surgical complication as well as decreased pain to the patient [22]. With the advent of LPHR, however, several questions arose. Some authors [1,23-25] have found 42% recurrence rates associated with laparoscopic repair at 18 month follow-up by upper GI barium swallow study. Interestingly, more than half of these patients with recurrence were asymptomatic. In contrast, open repair by abdominal or thoracic approach boasts a 15% recurrence rate at 35 months[1]. This data suggests significant opportunity for improvement in LPHR outcomes.
One such topic for discussion regarding LPHR includes antireflux procedures performed after repair of the hernia. Type 3 PEH classification implies that the gastroesophageal junction has migrated above the diaphragm, which may result in insufficiency of the lower esophageal sphincter with secondary gastroesophageal reflux symptoms. Therefore, in some institutions, antireflux procedure by fundoplication is a mainstay of LPHR. Draaisma et al. [22] however, suggests that the dissection of the stomach and esophagus down to the level below the diaphragm will suffice to control these symptoms. Little evidence regarding this assumption as a randomized controlled study has been brought up and up to now the question of appropriate anti-reflux protocol remains [26-30].
Another issue that remains unaddressed is the need to routinely perform esophageal lengthening during LPHR, given the finding that most patients present with a short esophagus [20,31-34]. Finally, the use of prosthetic mesh to repair the diaphragmatic crura remains questionable. In our early experience [35], we described the repair with Gore Tex graft, however others have reported erosion into the esophagus with nonabsorbable mesh. Current practice includes the use of biological mesh, but the procedure remains controversial [36-39].
While the prevalence of PEH in the adult population is about 5% [22,40], the literature shows that this prevalence increases 3 folds with age, particularly after 40 years [41-43].
The goals of this study are: 1) review what we know nowadays about surgical treatment of Parahesophageal hernia and 2) given that this hernia is more prevalent in the geriatric population and that this population is more prone to complication we focus on what can be eliminated from the surgery to make it more efficient and potentially avoid possible complication without compromise the success rate.
This is an IRB approved retrospective study of patients undergoing laparoscopic paraesophageal hernia repair from June 2009 to December 2013 at our hospital. We considered all the patients who presented in the office or in the hospital with type 2,3 and 4 hiatal hernia; confirmed by imaging such as CT and with reported symptomatology.
A summary of standard symptoms was obtained from the clinic notes prior to surgery. We graded severity of heartburn, regurgitation, chest pain, dysphagia, respiratory symptoms, and anemia (Table 1). All the patients additionally underwent standard preoperative tests, including an esophagogastroduodenoscopy and upper gastrointestinal series. A 24- hour pH study was not routinely obtained as in this type of hernia is not always accurate. White blood cell count, metabolic panels, and lactic acid values were obtained.
Grade |
||||
Current Symptoms |
1 |
2 |
3 |
4 |
Pyrosis |
No |
Occasional |
Moderate therapy |
Severe constant |
Regurgitations |
None |
Occasional (meal) |
Moderate (meal) |
Severe constant |
Dysphagia |
None |
Occasional (coarse food) |
Cleared with Liquids |
Severe liquids and solids |
Chest Pain |
None |
Occasional |
Frequent |
Continuous |
Pulmonary |
None |
Cough |
Asthma |
Dyspnea |
Anemia |
None |
Chronic |
Recent |
Active bleeding |
Table 1: Symptoms assessment.
All surgeries were performed laparoscopically through an abdominal approach in a standardized format. The surgery was done with 5 ports; 4 ports of 5 mm and 1 port of 12 mm was positioned in the American lateral position. The surgery began with visualization of the diaphragm and the left crura. Dissection began on the left side first with dissection of the stomach from the left crura, and then visualization of the pleura and dissection of remaining adhesions was performed. Next, the right crura was visualized, and the stomach and esophagus dissected away from remaining adhesions. The omental sac was visualized and dissected away from the esophagus as well. Routinely before the dissection of the esophagus with a 29-French endoscope was introduced into the esophagus as a stent; the endoscope additionally functioned to transilluminate the position of the esophagus and stomach into the operative field. This step was particularly important when the esophagus was very short and difficult to find. The next steps included complete dissection of the esophagus to bring it down below the diaphragm. Closure of the diaphragm with nonabsorbable stitches (either silk or Ethibond) was performed when possible otherwise if a mesh was needed, a bio-mesh, (Surgisis; Cook Surgery, Indianapolis, Indiana), was utilized. Finally, we performed a gastropexy in all our patients and we placed a gastrostomy tube only at the beginning of our experience.
The gastropexy was performed using non-absorbable stitches (2/0 silk). A full bite into the greater curvature of the stomach close to the antrum and a full bite into the abdominal muscle. Two to three stitches were used to secure the stomach to the abdominal wall.
If gastrostomy tube needed an 18 French gastrostomy tube was placed in the abdomen through a small incision. With bovie an opening was performed on the greater curvature of the stomach. A purse string was performed around the stomach opening with 2/0 Silk and the gastrostomy tube was inserted in the stomach and the purse string tied. Two extra stitches of 2/0 silk was then placed between the stomach and the abdominal wall to bring the stomach to the abdominal wall and then the balloon of the gastrostomy tube was inflated with 10 cc of normal saline. The tube was removed in the office after 6 weeks.
Hospital stay: All patients stayed for a minimum of 1 night in the hospital and on postoperative day number 1 they underwent an upper GI swallow to rule out intraoperative injury or post-operative leak.
DIET: After the upper GI series, the patient was started on clear liquid diet, advanced to a full liquid as tolerated. All patients were sent home on soft mechanical diet for 2 weeks till they came back in the office.
Follow up: Post-operative follow up was scheduled at 2 and 6 weeks, 3, 6, 12, 24 and 48 months after surgery. At follow up we performed a complete clinical examination and asked routine question according to the functional assessment (Table 2) as described in the literature [1,44]. Radiographic recurrence was defined as 1) symptomatology, 2) upper GI test with a paraesophageal herniation or proximal migration of the cardia. Sliding hiatal hernias were classified as less than about 5 cm, which was measured radiographically.
Total |
32 |
Men |
10 |
Women |
22 |
Median Age |
74 (60-89) |
Hernia Type |
|
Type 2 |
14 |
Type 3 |
12 |
Type 4 |
6 |
Esophagitis |
29 |
ASA Score |
|
IV |
11 |
III |
21 |
Table 2: Demographics.
The variables were compared by using the T test continuous variable. Comparison of recurrence was performed by using chis square test.
In 40 months we considered 32 patients; 10 males and 22 females (Table 2). Median age was 74 years with a range of 60 to 89. Patient population consisted of 14 type 2 hernias, 12 type 3 hernias, and 6 type 4 hernia. All the patients had esophagogastroduodenoscopy before surgery. Esophagitis was present in 29/32 patients. One patients showed mucosal changes on EGD. Preoperative lactate level, WBC were considered pre and postoperative (Table 3), the ASA score by American Society of Anesthesiologists was 4 in 11 patients and 3 in the remaining (Table 2). None of the patients were treated emergently as defined within the first 12 hours of presentation to the Emergency Department. Five patients were treated within 24 hours to admission of the emergency room and one patient within 48 hours of the admission. All the other patients were treated as an elective surgery.
|
Pre Op |
Post Op #2 |
P |
WBC |
7 ± 3 |
10 ± 2 |
ns |
Lactate |
2.1 ± 1.1 |
0.9 ± 0.8 |
ns |
Table 3: Laboratory Reports.
Twenty-eight patients underwent laparoscopic surgery with primary closure, 4 were closed with the BIOMESH as described in the method. Overall surgical results produced 25 patients with gastroeshopageal junction placement 2 cm below the diaphragm, 6 patients with GE junction at 1 cm below the diaphragm. Out of 32 patients, we placed gastrostomy tube in 3 cases; all the others received gastropexy. Average intra-operative time was 1 hour, 53 minutes.
There was no operative mortality and no postoperative mortality (Table 4).
Surgical Associated Points |
|
BIOMESH |
2 |
EG Junction After Surgery |
|
2 cm below |
12 |
2 cm below |
25 |
1 cm below |
6 |
0 cm below |
1 |
Gastrostomy Tube |
4 |
Gastropexy |
32 |
OR Time |
1 hr 53 minutes ± 20 minutes |
Mortality |
0 |
Morbidity |
|
Pneumothorax |
2 |
Bleeding |
1 |
Trocar Bleeding |
1 |
Pleural Effusion |
1 |
Recurrence |
1 |
Table 4: Intraoperative data-Repair measure of esophagus in abdomen.
Complications of the surgery included 2 episodes of intraoperative pneumothorax which resolved spontaneously by stopping the flux of CO2 , 1 episode of bleeding from the short gastric controlled with a stitch, 1 episode of bleeding from the trochar site, and 1 pleural effusion. Upper gastrointestinal series performed on post operative day 1 showed normal position of the gastroesophageal junction in 31 patients. No re-operations were done. One recurrence was found after 6 months presenting as a reflux and showed as a slinding hiatal hernia by upper endoscopy. This was the one patient with difficult to place the EG junction below the diaphragm.
The functional assessment of the patients showed markedly and significant improvement after surgery (Table 5).
Symptoms |
Preoperative (n) |
Postoperative (n) |
x2 test, P |
Pyrosis |
30 |
4 |
<0.001 |
Chest pain |
31 |
1 |
<0.001 |
Regurgitation |
6 |
0 |
<0.001 |
Dysphagia |
15 |
0 |
<0.05 |
Pulmonary |
21 |
0 |
<0.001 |
Anemia |
12 |
0 |
<0.001 |
ENT |
1 |
0 |
NS |
Cardiac |
1 |
0 |
NS |
Vomiting |
8 |
0 |
NS |
Table 5: Functional assessment: Pre- and Postoperative Patient’s symptoms. ENT indicates ear, nose, throat; n: number of patients presenting the symptom; NS: not significant; Pulmonary indicates pneumonia or infection recurrent.
The evaluation of laparoscopic PEH repair is complex, as multiple variables affect the evaluation of post-operative outcomes. For example, recurrence evaluation may be studied in the context of radiological evidence versus clinical signs or symptoms. In addition, surgical procedures for PEH repair vary in the use of mesh, gastropexy, and gastrostomy placement. We will discuss these factors in addition to others in order to further understand the success and complexities of LPH repair.
Treatment of paraesophageal hernia repair has evolved over time. The incidence of recurrence rates has been varied over time and with different technique of hernia repair with mesh or without and with pexy as reported in table 6.
Authors |
Recurrence rate % |
|
Edye [23] |
4 |
|
Wu [25] |
6 |
|
Dellemagne [1] |
42 |
|
Witchterman [3] |
7 |
|
Diaz [17] |
22 |
|
Maziak [28] |
13 |
|
Frantzides [37] |
0 with mesh |
22 with suture repair hernia |
Ganderath [45] |
8 with mesh |
26 with suture repair hernia |
Poncet [40] |
10 with pexy |
50% without pexy |
Table 6: Recurrence rate after PHE Surgery.

Figure 1: Incidence of presenting symptoms.
In 2000, Dallemagne [1] reported a 42% incidence of radiographically recurrent hiatal hernia at 17 months after laparoscopic repair in a series of 21 patients; in addition, other authors have reported varying rates throughout the literature, from 7-55% [3,14-17,25,45-53]. Additional question remains over the exact recurrence rate of paraesophageal hernia after surgery given that most of the recurrences are clinically asymptomatic and often remain undetected [25,45-46]. Dallemagne [1] conducted an additional, larger study of this subject with a 10 year follow-up period and reported an overall 66% recurrence rate. In summary, it is imperative to discuss the variable procedures and follow-up protocol found throughout the literature in order to understand the significance of this data.
As mentioned above, Dallemagne [1] used follow up of clinical symptoms and radiological assessment with barium esophagogram at an average of 10 years post-op, and confirmed a 66% recurrence rate. This recurrence rate followed a standard operative procedure which included the following: 1) complete excision of the peritoneal sac within the mediastinum, 2) meticulous reduction near the stomach, 3) repair of the diaphragmatic hiatus, and 4) elimination of tension by manipulation above the gastroesophageal junction in order to lengthen the esophagus. Tension at the hiatus is known to shorten the esophagus and impair full hernia repair. In fact, our experience has found lengthening the esophagus quite difficult, as also noted by Draaisma [22]. To help in the dissection we found that placement of and oral endoscope during the operation can help guide the high and posterior esophageal dissection particularly in the elderly population where the tissue is easy to tear. The endoscope allow to: 1) feel the esophagus when it is compressed and up in the chest, 2) trans illuminate the esophagus avoiding esophageal injury, 3) minimize the dissection around the esophagus.
Furthermore, Maziak, Todd, and Pearson [28] reported a 13% recurrence rate in patients that did not undergo intra-operative elongation of the esophagus, also known as the Collis procedure. The criteria for performing Collis esophageal lengthening remains questionable throughout the literature. Altorki and colleagues [54] additionally showed that 90% of the patients do better with Collis lengthening, noting that the majority of patients requiring paraesophageal repair have a short esophagus. Draaisma [22] reports the same. However, others reported [7] a short esophagus in 2% on the patient, only 2 patients out of 55 and therefore they do not believe that a Collis procedure is required for successful repair. Our clinical experience agrees with Horvath, et al. [55], which showed that a short esophagus is very common in this patient population. For authors that did an extensive laparoscopic esophageal mobilization without a Collis procedure, the recurrence rate was 66% [1,56]. Moreover, Lubezky and Altorki [52,54] reported that with a thoracic approach to surgically release a short esophagus, the recurrence remains 5% regardless of Belsey or Nissen fundoplication without a lengthening component. Overall this data suggests that surgeons should attempt to intra-operatively elongate a short esophagus before performing fundoplication. Our only recurrence was in the patient that we had difficulties to bring the EG junction below the diaphragm and we did not performed the elongation of the esophagus since the esophagus was very friable.
No mesh was used in our patient that had recurrence. Mesh is another important variable in PEH repair is the use of mesh to close the defect in the diaphragmatic hiatus. Lubezky [52] found that mesh is associated with significant increase of re-operation or recurrence; therefore we avoided the use of mesh in our cases when possible. In addition, Stadlhuber [57] showed that mesh increased the requirement of potential esophagectomy for erosion into the esophagus. In contrast, others [24,45,57] reported mesh might reduce, but not completely eliminate the risk of PEH recurrence. However, this is shown with the use of non-absorbable mesh, which leads to possible erosion in long term follow up [1,22,58].
In our personal experience of repairing PHE with non absorbable mesh, we had great result the first year but towards the 12 months we started seen some erosion of the mesh into the esophagus. That experience made us think about other option such as no mesh at all or a more pliable mesh and that was the reason we turn towards absorbable mesh.
In a large series produced by Poncet [40], patients with type 3 hiatal hernia treated without mesh had very good results at 56 months follow up; reporting good functionality early, with a minimal morbidity and mortality. Multiple authors have shown lower PEH recurrence rates by adding a prosthetic patch with cruroplasty; demonstrating a 9% recurrence rate versus 24% recurrence without the patch [37,45,59,60]. Frantzides [37] reports a 22% recurrence in a group with simple sutures versus 0% in a group with prosthetic patch (type of patch here). The Ganderath series also found a 26% recurrence rate in a simple suture group versus 8% in prosthetic patch group (Ganderath series) [45] (Table 6). However, a short follow limits these results up of 1 to 2.5 years in this series. Furthermore, this series [40] showed a significant higher postoperative dysphagia rate in the prosthetic mesh group. In addition, [57] reported 28 cases of complication after prosthetic closure of the hiatus with the use of both non-absorbable and biological mesh. This complication usually occurred within 18 to 24 months, and complications such as re-operation for retroesophageal erosion, including esophagectomy and gastrectomy were reported. Weight loss, sepsis and abscess are also reported when using a patch [12,31,59,60]. This manoeuver effectively reduces intra-operative complications [13,59,61].
We used biosynthetic mesh in 4 cases; all the patients had no postoperative complications. In our experience, mesh is a useful tool when dealing with a large diaphragmatic hiatus. Unfortunately, the verdict is still out to show which mesh should be used in this operation and further studies are needed.
In an experienced hand, laparoscopic PEH repair has a very low conversion rate to an open procedure. The conversion rate reported in the literature is between 4.5% [40] and 4.2% [22]. The average morbidity is about 7.8% [40] in laparoscopic cases versus a 16.2% in open cases [22]. Another interesting point is that the rate of PEH repair has increased with the advent of laparoscopic surgery. Well known advantages of laparoscopic procedures, including decreased postoperative complications, hold true for PEH repair. In addition, elective laparoscopic repair can prevent emergent repair of PEH, which is associated with a higher rate of gastric mucosal damage, perforation, and hemorrhage [12,28,57,62]. We did not have to convert any patient to open repair. In the hand of a trained laparoscopic surgeon the conversion should be minimal to zero.
Interpretation of preoperative manometry of esophageal motility and GE junction integrity remains difficult in PEH patients because of distortion of the normal anatomy. With the stomach lying above the diaphragm, we may not fully rely on this test as a perfect indicator of motility. We then must examine the role of anti-reflux fundoplication in PEH repair. Spechler [43] also suggests that all PEH repair patients should undergo fundoplication because preoperative evaluation of motility is unreliable. Ponsky [63] reports that routine use of Nissen fundoplication should be used when pre-operative esophageal motility is normal. Furthermore, Poncet [40] also reports that fundoplication decreases symptomatology and additionally rejects association between the wrap of the Nissen fundoplication and postoperative esophageal stenosis. Poncet argues that perioperative difficulty in esophageal mobilization and postoperative swelling or stenosis are related to tight diaphragmatic anatomy. Geha [27] also reported favorable outcomes of fundoplication in PEH repair, showing that it prevented the incidence of postoperative gastroesophageal reflux.
There is only one randomized control trials published regarding antireflux procedures in PEH repair. In this paper the authors conclude that: “Laparoscopic repair of PEH should be combined with a fundoplication to avoid postoperative gastroesophageal reflux and resulting esophagitis. Fundoplication-related side effects do not appear to be clinically relevant. Multicenter randomized trials are required to confirm these findings.” But these were initial results and they suggest other study to confirm it. In our cases, we did have reflux, but we were able to controlled with medications. Moreover the population described was younger than ours. In more senior population we postulate that fundoplication can be use as needed, following reason: 1) impossibility to have a reliable manometry preoperatively, 2) does not add to the pathophysiology of the disease which is the volvulus of the stomach and not the reflux. Therefore, if we need to choose on procedure and minimize our time and effort gastropexy is more important than fundoplication since does not allow any movement of the stomach while fundoplication allow still an 180^ rotation.
This is a sensitive subject giving the fact that create multiple academic discussion. In reality the PHE do not present with reflux in the majority of the cases, they might develop GERD after the surgery with a alignment of esophagus and stomach. As we are learning from our colleagues and surgical and GI meeting the rate of antireflux surgery are decreasing around the country since the introduction of the proton pump inibhitor. So, in our patients the question came natural as to why perform another surgery which will prolong the anesthesia time and the risk in senior citizen patient while instead can be avoided and treated with medication? Based on the understanding of the pathophysiology of the PHE we decide to avoid antireflux procedure when possible to minimize the procedure. We think about the problem of the PHE is the rotation of the stomach, that is our goal to fix! Therefore, the fixation of the stomach to the abdominal wall is the single most important manoeuver to perform.
Gastropexy remains an additionally important issue to discuss. For example, Poncet [40] reports a 50% recurrence rate in a group without gastropexy versus 10.8% in a group with gastropexy. Performing anterior gastropexy therefore can significantly reduce the post operative recurrence rate, which has been already reported in a different series [17,63-67]. By anchoring the stomach anteriorly, we can prevent re-entry of the stomach into the mediastinum. Therefore, anchoring the stomach by stitching the stomach to the muscle, and/or placing a gastrostomy tube when it is needed is probably the best method to avoid malrotation while additionally decreasing the risk of gastric volvulus and strangulation. We performed gastropexy in 100% of our patients and we did not have any recurrence of the stomach in a position above the diaphragm or volvulus. We placed only three gastrostomy tube in our early experience and then we notice that the gastropexy was sufficient.
Further complexities arise when we discuss the aforementioned issues in the context of a geriatric patient population. For example, Coelho [43] reviewed hiatal hernia repair in patients with a mean age of 77 years, and reported significant postoperative complications such as, leak, nausea and vomiting. We attempted to avoid these issues by limiting hospital stay to a mean of 24 hours in order to decrease the likelihood of both delerium and health care acquired infection. Kercher [58] considered PEH repair in 49 patients with a mean age of 78 years. The patient population mirrored ours in many ways. Patient comorbidities included coronary artery disease, CHF, atrial fibrillation, hypertension, COPD, cerebrovascular disease, and history of thrombotic events; all with an ASA score of 3. Kercher [58] however, placed a gastrostomy tube (GT) tube in the majority of patients, with removal at 2 months postoperatively. GT placement has complications such as leak, accidental displacement, and infection. We avoided GT placement if possible by placing 2 gastropexy stitches from the full serosa and muscle of the stomach to the abdominal wall in a circulatory fashion. We had 1 recurrence as hiatal hernia.
Overall, complete comprehension of all the potential complications of PEH repair in geriatric populations is quite difficult due to both varied procedures used to repair and pre-existing medical co-morbidities. Follow-up is therein impaired by patient morbidity and mortality associated with these factors.
If we just look at our population and in general of the population with these hernia, we noticed that they are senior. The goal of the surgery in our opinion is to avoid complications by take away the change of a possible stomach volvulus first and minimize the manouvre and the anesthesia time in all. That is why we think that some of our suggestion can help the patient in the long run even if we do not follow the way this is thought to be done.
We tend to repair the diaphragm primarily to decrease the placement of a foreign body (mesh) and possible erosion of the esophagus in the geriatric population and a gastropexy on all.
It is important to note, however, that the aging population will increase the prevalence of PEH in surgical patients over time; treatment of this disease in this population must be addressed keeping in mind their quality of life.
PHE is a disease more prevalent in the geriatric population. Given this we need to operated careful and eliminate all the steps which are not needed.
In our study and literature review, we conclude that: 1) Mesh should be used only when the crura cannot be closed primarily with stitches such as large defect till we all agree on the best mesh to use absorbable or not; 2) gastrostomy tube or always a 2 full thickness gastropexy stitches to reduce the risk of PEH recurrence and gastric volvulus it is the most important manouvre if we think exclusively at the phatophysiology; 3) effort should be made to safely lengthen the esophagus off of underlying adhesions for proper GE junction placement. We suggest placing and endoscope to guide the esophageal dissection; 4) adding the antireflux procedure is not always necessary.
Therefore we suggest to minimize the minimal invasive procedure by eliminate surgical manoeuvre or minimized having always in mind the patient quality of life.
Download Provisional PDF Here
Article Type: Research Article
Citation: Frezza EE (2017) Laparoscopic Paraesophageal Hernia Repair and State of Art in Geriatric Populations and Our Experience with 4 Years follow up. How Can We Minimize Our Minimal Invasive Procedure? J Gastric Disord Ther 3(1): doi http://dx.doi.org/10.16966/2381-8689.131
Copyright: © 2017 Frezza EE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access