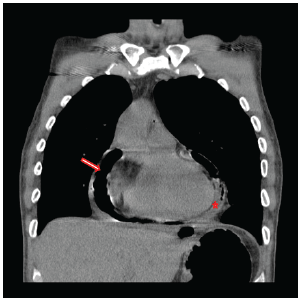
Figure 1: Chest CT documenting gross pneumopericardium (arrow) with a concomitant pericardial effusion (star) prior to the initial operation to address the gastropericardial fistula.

Granchi T Wierzbicki J Wideroff M Nau P*
200 Hawkins Drive, lowa City, Iowa, USA*Corresponding author: Nau P, 200 Hawkins Drive, lowa City, Iowa 52252-1086, USA, Tel: 319- 356-7675; Fax: 319-356-4609; E-mail: peter-nau@uiowa.edu
A 42-year-old male presented with symptoms of a gastrointestinal bleed and suspected recurrent gastropericardial fistula. Six years prior, he had achieved resolution of his reflux symptoms after an uncomplicated laparoscopic Nissen fundoplication. The post-operative course was complicated by the development of a recurrent gastropericardial fistula necessitating two distinct operations. The patient presented in extremis with symptoms of a gastrointestinal bleeding after endoscopy reportedly showed an ulcer in the region of the previous fistula. The patient was emergently explored and a fistula from the gastric fundus to the left ventricle of the heart was discovered. Takedown of the gastrocardiac fistula was performed and the patient recovered uneventfully thereafter. This report of a gastrocardiac fistula highlights the importance of the therapeutic tenets utilized to recognize and treat this rare complication.
Gastrocardiac fistula; Laparoscopic Nissen fundoplication
Symptomatic gastroesophageal reflux disease (GERD) refractory to medical management requires more invasive methods of treatment. The surgical option most frequently employed is the Nissen fundoplication. This procedure has proven cost effective with better symptom resolution than medical treatment and with an acceptable safety profile [1-3]. Postoperatively, patients may experience transient complaints of bloating and dysphagia. An extremely rare complication is a gastropericardial fistula. Herein is a case presentation of an individual who underwent Nissen fundoplication for refractory GERD complicated by a gastropericardial and, ultimately, gastrocardiac fistula. This later complication has not previously been reported in the literature.
A 42-year-old male presented in March of 2015 with a 48 hours history of melena, a hemoglobin of 7.8 and malaise. He was transferred to the University of Iowa Hospitals and Clinics (UIHC) with hypotension and tachycardia. The source of his bleed was a suspected recurrent gastropericardial fistula. His surgical history was significant for several previous foregut operations. He initially underwent a reportedly uncomplicated laparoscopic Nissen fundoplication at an outside facility in 2009 for GERD refractory to maximal medical treatment. Postoperatively, he described good resolution of his reflux symptoms and esophagitis. Four years after his index operation he presented with chest pain and a leukocytosis. He was diagnosed with acute pericarditis based on an abnormal electrocardiogram (EKG). A follow-up echocardiogram was completed which demonstrated pneumoarcdium. This finding was confirmed on chest CT (Figure 1) and showed extensive pneumopericardium and pericardial effusion. This was addressed by his local surgeon with a subxiphoid pericardial window, fistula takedown with primary gastric repair and concomitant antibiotic administration. Nine months after this revisional procedure, the patient presented to his local hospital with a perforated duodenal ulcer likely secondary to high dose nonsteroidal anti-inflammatory drug use. This was treated with an exploratory laparotomy and Graham patch. Three months later, the gastropericardial fistula was noted to have recurred (Figure 2). Due to this persistent fistulous tract, he was taken back to the operating room for a primary suture repair with omental patch interposition and revision of the Nissen fundoplication. Three months after that procedure, the patient presented to an outside facility with melena, low hemoglobin and malaise. Endoscopy reportedly showed a visible ulcer in the region of the previous gastropericardial fistula. Following six days of nonoperative management which included serial hemoglobin monitoring, transfusions and repeated endoscopies, the bleeding failed to stop and he was transferred to the UIHC for definitive care.

Figure 1: Chest CT documenting gross pneumopericardium (arrow) with a concomitant pericardial effusion (star) prior to the initial operation to address the gastropericardial fistula.
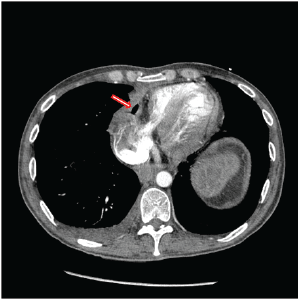
Figure 2: Chest CT prior to second revision of gastropericardial fistula documenting persistent pneumopericardium (arrow).
Upon arrival, the patient became hypotensive and tachycardic. Due to the history of a perforated ulcer, the patient underwent emergent endoscopy in an effort to identify the bleeding source. This was notable for a large volume of old, clotted blood within the antrum and fundus as well as fresh blood filling from an uncertain, proximal location. Given the, clinical picture the patient was resuscitated and taken to the operating room for an emergent exploration.
Upon entrance into the abdominal cavity, a wide gastrotomy was made and the old blood removed. Active arterial bleeding was identified from the proximal stomach. This was unable to be controlled with temporizing suture ligatures. The stomach was packed and a mediastinal exploration was performed through a median sternotomy. Operative findings included dense adhesions consistent with previous pericarditis and an obvious fistula from the gastric fundus to the left ventricle. The defect in the myocardium was repaired with interrupted pledgeted sutures. The fundoplication was completely taken down to facilitate visualization. Due to the proximity to the esophagus and tissue edema, a primary repair of the 2.5 cm fundus defect was performed (Figure 3). Given the acuity of the patient, a repeat fundoplication was deferred. The diaphragm was repaired with a piece of biologic acellular dermal matrix. Postoperatively, the patient completed a ten day course of broad spectrum antibiotics with fungal coverage. He had an uneventful recovery and was discharged in good condition two weeks later. He was seen four months post-operatively and was tolerating a regular diet without difficulty and was regaining strength.
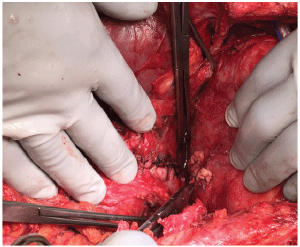
Figure 3: Intra-op image of the gastric repair with interrupted 2-0 silk sutures on the left and the pledgeted ventricular repair with 3-0 monofilament suture on the right.
The surgical treatment of GERD has been shown to be durable, cost effective, and with better quality of life outcomes than medical management alone [1-3]. There are numerous surgical options for the treatment of GERD including transoral incisionless fundoplication, magnetic sphincter augmentation (MSA) and the classic Nissen fundoplication [1,4-7]. In high volume centers, the major complication rate for Nissen fundoplication is less than 2% and the rate of early reoperation less than 1% [8,9].
The complication of a gastrocardiac fistula is previously undescribed. There are several case reports in the literature of fistulous formation between the gastric conduit and the pericardium, aorta, or bronchi complicating resectional procedures for malignancy [10-13]. A fistula between the neo-esophagus and myocardium following esophagogastrectomy occurs much less frequently [14-17]. To date, there are four reported cases of gastropericardial fistulas presenting as late complications of laparoscopic Nissen fundoplications (Table 1) [18-21]. Clinical presentation of gastrocardiac fistulas may be misleading with both abdominal and chest pain as well as more ambiguous complaints such as malaise, tachycardia and nausea [18,19]. Rapid diagnosis and expeditious operative intervention are critical to the survival of the patient. Prompt evaluation with CT using water soluble contrast to assess for pneumopericardium or a pericardial effusion is a reasonable first step. Additional diagnostic tests which can be considered based on the stability of the patient include needle aspiration of a pericardial effusion or gastroscopy with judicious insufflation for direct visualization of the fistula.
Author, year |
Interval from Anti- |
Presenting complaint |
Wrap Migration? |
Su-Gandarilla, 2000 [21] |
5 years |
Substernal pain |
Yes, proximal herniation |
Murthy, 2002 [20] |
11 months |
Severe abdominal pain, fevers, and weight loss |
Yes, proximal herniation |
Sihvo, 2006 [19] |
7 years |
Right-sided chest pain, hypotension |
Yes, proximal herniation |
Pop, 2007 [18] |
7 years |
Malaise, fever, cough, and tachycardia |
Yes, proximal herniation |
Table 1: A table of the previous reports of gastropericardial fistulas following laparoscopic Nissen fundoplication including salient characteristics.
Gastropericardial fistulas may present in both an early and late setting. Early fistulas often occur in the setting of re-operative surgery and procedures complicated by subphrenic abscesses. Conversely, late fistulas are typically associated with a recurrent hiatal hernia and concomitant gastric ulcer formation [19]. In three of the four aforementioned cases, gastric ulcers were clearly associated with the pericardial fistula [18- 20]. In all four previous reports the fundoplication had herniated into the thoracic cavity. In this case, the timing was consistent with an ulcer complicating a recurrent hiatal hernia. However, on re-exploration there was no recurrent hernia. Instead, there was an inadequately addressed, recurrent gastropericardial fistula and associated ulcer which preceded the erosion into the myocardium. It has been proposed that improper hiatal closure and/or tension on the wrap may lead to thoracic migration of the fundoplication [18-21]. The fact that proximal migration of the fundus into the chest was noted in all of the previous case reports underscores the importance of adequate esophageal mobilization and proper hiatal closure. Whether it is an ischemic insult, protracted mechanical forces or another etiology, a chronic gastric ulcer in a herniated wrap appears to play a central role in the development of this uncommon complication. The ability to prevent proximal migration of the wrap into the chest and creation of a tension-free fundoplication is critical in the avoidance of ulcer formation and this devastating complication.
The diagnosis of this case was complicated by the history of a perforated duodenal ulcer, necessitating an endoscopy for characterization of the location of bleeding. In a setting in which the diagnosis was less ambiguous, it can be argued that an endoscopy may exacerbate the situation with tamponade physiology. This was certainly considered with this patient. However, with conservative use of gastric insufflation the gastroscopy was completed safely. This approach has been effectively used by others [8,9]. In this case the success was likely secondary to the small defect in the myocardium and the intense fibrosis between the pericardium and myocardium effectively limiting the ability of gas to reflux into the pericardial sac. Given the severity of this patient’s reflux, it would’ve been preferable to create an anti-reflux barrier with a fundoplication. However, due to the acuity of the case and the massive resuscitation, a redo fundoplication was considered unsafe and was therefore deferred. Perhaps more important than an anti-reflux procedure was the adequate mobilization of the esophagus and secure crural repair so as to prevent re-herniation of the stomach as well isolate the healing myocardium and stomach from each other.
Surgical treatment of GERD is an effective option with an acceptable safety profile for those with medically refractory disease. Gastropericardial fistula formation following Nissen fundoplication is an uncommon event with an often ambiguous presentation. When confronted with a patient in whom this diagnosis is on the differential, evaluation with contrast enhanced CT is necessary. Alternative procedures such as endoscopy and pericardiocentesis are useful adjuncts if hemodynamic stability permits. Surgical tenets of treatment include adequate drainage of the pericardium, excision of the fistulous tract, and secure fixation of the stomach within the abdominal cavity. In this case, the gastropericardial fistula was initially treated inadequately resulting in a gastrocardiac fistula. For this patient expeditious operative intervention and aggressive resuscitation facilitated a successful outcome.
GT, WJ, WM, NP all participated in the drafting and editing of this manuscript.
Download Provisional PDF Here
Article Type: Case Report
Citation: Granchi T, Wierzbicki J, Wideroff M, Nau P (2016) Laparoscopic Nissen Fundoplication with delayed Gastrocardiac Fistula: Report of a Case and Review of the Literature. J Gastric Disord Ther 2(5): doi http://dx.doi.org/10.16966/2381-8689.129
Copyright: © 2016 Granchi T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access