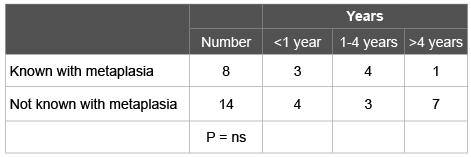
Table 1: Years between the endoscopy without oesophageal cancer and the previous procedure
RJLF Loffeld*
Department of Gastroenterology and Internal Medicine, Zaans Medisch Centrum Zaandam, The Netherlands*Corresponding author: RJLF Loffeld, Department of Gastroenterology and Internal Medicine, Zaans Medisch Centrum, PO BOX 210 1500 EE Zaandam, The Netherlands, Tel: 31-75-6502779; Fax: 31-75-6502379; E-mail: loffeld.r@zaansmc.nl
Background: Guidelines recommend surveillance of Barrett’s oesophagus (BE). Patients with oesophageal adenocarcinoma are often not known to have pre-existing BE.
Aim: Assess how many patients with oesophageal adenocarcinoma were previously diagnosed with BE, and the burden of diagnosing one cancer in BE surveillance?
Methods: All patients with oesophageal adenocarcinoma in the period 2001-2010 were studied. Endoscopy records and pathology reports were searched for results of prior endoscopy. Also all patients diagnosed with BE in the period 1992 - 2013 were used as a mode of estimating the burden of surveillance.
Results: Oesophageal adenocarcinoma was diagnosed in 103 patients. Of these 16 (15.5%) had prior endoscopy. Of these 8 (7.7%) had earlier BE. In the period of 22 years 1134 (3.5%) endoscopies revealed BE in 831 patients. In 25 cases BE together with adenocarcinoma was seen. Assuming that 50% of patients would have died because of different reasons or refused surveillance, 525 extra endoscopies would have been done in order to detect one cancer.
Conclusion: The majority of patients with oesophageal adenocarcinoma are not known to have prior BE. The number of patients developing cancer seems high but the burden to detect one curable cancer is much higher.
Oesophageal cancer; Barrett’s; Metaplasia; Guidelines; Surveillance
Prevalence of adenocarcinoma of the oesophagus is increasing, although the absolute yearly figures are still rather low. In 2002, 1308 patients were diagnosed with oesophageal adenocarcinoma in the Netherlands, this figure rose to 1823 in 2012 [1]. Metaplastic epithelium in the oesophagus (Barrett’s oesophagus, BE) is a well-known complication of long standing reflux disease. Especially BE with dysplasia is considered a pre-cancerous lesion. Removal of dysplastic epithelium can prevent cancer [2].
Development of metaplastic epithelium in the oesophagus in fact is “protective”. Metaplastic epithelium is more resilient against the detrimental effects of acidic stomach contents. Patients with BE experience less reflux complaints [3]. However, absence of symptoms is not associated with a lower risk of malignant progression [4].
Because of the malignant potential regular surveillance is recommended [5]. Guidelines are based on the assumed annual risk of oesophageal cancer of 0.5%. Adherence to the surveillance protocols poses a heavy burden, not only on patients but also on endoscopy and pathology departments.
In normal daily practice oesophageal adenocarcinoma often is the first presentation of complicated intestinal metaplasia. The majority of patients were not known to have a prior diagnosis of BE [6,7].
For this reason, a study was done in consecutive patients with adenocarcinoma in the oesophagus in order to assess the number of patients with previously diagnosed BE or had undergone endoscopy without BE. In addition, the number of endoscopies necessary in order to detect one cancer in surveillance was calculated.
All consecutive patients diagnosed with oesophageal adenocarcinoma, in the Zaans Medisch Centrum, the community hospital of the Zaanstreek region in the Netherlands, during a period of ten years (2001-2010) were studied. Endoscopy records and pathology reports were searched for results of prior upper gastrointestinal endoscopy. In addition, in order not to miss any patients with prior BE, the nationwide pathology database of the Netherlands was searched for possible oesophageal biopsies done in other hospitals.
In addition, all patients diagnosed with BE (this is Barrett’s seen in the first endoscopy in a patient) in a period of 22 years (1992-2013) were used as a mode of estimating the endoscopy burden of surveillance.
The endoscopic procedure was done with Olympus endoscopes (Olympus Nederland BV, Zoetermeer the Netherlands). In 1992 fibre optic endoscopes were used, from 1993 the EVIS 100 video endoscopes were introduced. Since the beginning of 2000 this system has been replaced by the EXERA 160 and 180 system of Olympus. Since 2011 also endoscopes from Fujinon are used.
The results of the procedures were noted in a written standardized report. From 2003 a custom-made computerized system was used (Endobase™ Olympus).
With modern endoscopes the macroscopic identification of BE is reliable. Biopsy specimens of the oesophagus, to confirm the diagnosis, were not routinely done.
The diagnosis of oesophageal cancer was made endoscopically, and confirmed histologically, if the majority of the tumour was visualized in the distal oesophagus and the gastric cardia was free of tumour during inspection in U-turn.
Statistical analysis was done with chi-square test for contingency tables. A p-value below 0.05 was considered statistically significant.
In the study period 16996 upper gastrointestinal endoscopies were done. Oesophageal adenocarcinoma was diagnosed in 103 patients (82 men, 21 women). Of these 16 (15.5%) had undergone prior upper gastrointestinal endoscopy (12 men, 4 women). Hence, 84.5% of the cases was diagnosed in patients unknown with BE. Of the patients in whom an endoscopy was done in the past, 8 (7.7%) had earlier BE. Table 1 show the time elapsed in these patients between the endoscopy with and without cancer. There was no significant difference between patients known or not known with BE. One of these 8 patients participated in a nation-wide surveillance study. He had no dysplasia but developed a small cancer within one year after the surveillance endoscopy. Of the patients known with prior BE three underwent curative treatment; five were inoperable due to metastatic disease at time of presentation.

Table 1: Years between the endoscopy without oesophageal cancer and the previous procedure
Out of the total number of consecutive endoscopies in the period of 22 years (31816) 1134 (3.5%) revealed BE. BE was seen in 831 patients. Figure 1 shows the yearly detection of BE in this period of time. This makes an average of 38 patients each year with newly diagnosed Barrett’s (excluding the patients with incident Barrett’s and cancer, n=25). Assuming that a conservative estimate of 50% of patients would have died because of co-morbidity or refused surveillance, the number of extra endoscopies in order to detect one cancer would have been 525.
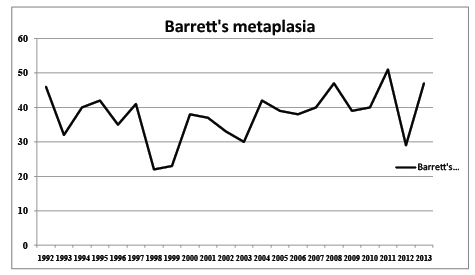
Figure 1: Yearly incidence of BE in daily endoscopy practice
Metaplastic epithelium in the oesophagus is the result of long-standing reflux. This metaplastic epithelium is more resilient to the acidic contents of the stomach. Development of metaplasia could be considered as a protective mechanism of the oesophagus. There are three different types of metaplasia: the cardia type, the oxyntic type and the intestinal type characterized by goblet cells. Only the latter, with probably the lowest incidence, is pre-cancerous. The change of developing cancer in nondysplastic Barrett oesophagus is only 0.12% to 0.38% per year [8].
Several guidelines recommend endoscopic surveillance. The goal is detection of high grade dysplasia and even cancer in an early and thereby treatable stage. Endoluminal therapy is successful. However, intestinal metaplasia may return in one-third of cases and this makes ongoing surveillance needed [2]. Although newer studies report a lower recurrence percentage using radiofrequency ablation [9,10].
Grant et al. [11] compared the tumour stage and survival within a surveillance program versus those who presented with prevalent disease. Patients within the surveillance program had better survival and were less likely to undergo surgery than those who presented with prevalent disease. The improved survival was not secondary to lead time bias.
Thus, many patients with Barrett’s have to undergo numerous endoscopies. But endoscopic treatment of non-dysplastic Barrett metaplasia as a means of preventing cancer is questionable [8]. Recent studies have doubts due to the new, lower estimates of the rate of progression of Barrett’s oesophagus to cancer [12]. Hvid-Jensen et al. [13] did a population-based study. They identified patients with Barrett’s and did an analysis over a period of 5.2 years. The yearly risk of oesophageal adenocarcinoma was 0.12% (95% CI, 0.09 to 0.15). This absolute annual risk of 0.12%, is lower than the risk of 0.5%, which is the basis for current surveillance guidelines.
Columnar lined epithelium is present in up to 25% of GERD patients. If all these patients had to undergo surveillance this would cause a significant rise in costs [14].
The present study is in accordance with a recent study showing that the majority of adenocarcinomas occur in patients not known to have preexisting Barrett’s [7]. If the number of new patients with BE is taken into account and one believes that surveillance can prevent development of cancer many patients have to undergo endoscopy with the result that a minority of cancers is detected at the price of a large burden of repeated endoscopies including all histological examinations.
Compliance with the guidelines is far from optimal. In a study by Menezes et al. [15] a questionnaire was used in order to study the adherence to the guidelines which was found to be 79-86%. The response rate in this study was low, namely 24%. The reason for the variability in adherence to guidelines was not clear. Results of specific trials cannot always be extrapolated to daily practice. This could be a reason for not adhering to guidelines.
The macroscopic diagnosis of Barrett’s with video endoscopes is easy. Ulceration within the Barrett’s segment and long-segment metaplasia is associated with an increased risk of progression [4].
After explaining surveillance in cases of BE with normal smooth surface, the odd’s ratios of developing cancer and the burden of follow-up endoscopy, some patients in usual daily practice agreed to undergo regular endoscopy. However, the majority of patients choose a wait and see policy without regular surveillance. Also life expectancy and co-morbidity of the individual patient were taken into account.
The results of the present study show that the majority of patients with oesophageal cancer never underwent prior endoscopy, nor were known to have Barrett’s epithelium. Only 7% of patients with cancer had a prior endoscopy in which Barrett’s was seen. Three of these patients developed cancer within one year.
Even in case of regular surveillance the three detected cancers would have been missed, hence these can be considered interval cancers and failure of surveillance.
Gordon et al. [16] studied the costs of surveillance. They compared three groups of patients: no surveillance, 2-yearly surveillance, and 6-monthly surveillance. Endoscopic surveillance resulted in extra costs per QALY ratio of $60,858. They concluded that endoscopic surveillance of patients with non-dysplastic BE is not cost-effective.
On the basis of current epidemiological data [15], surveillance would never have become clinical practice. The number of patients developing cancer seems high but the burden to detect one curable cancer is much higher. The very low number of cancer in patients with Barrett’s in daily practice challenges the necessity of regular surveillance. It can be questioned whether the effort in a large group of patients is really worthwhile in this time of lowering budgets in health care. Better selection, using biomarkers, could identify patients in whom surveillance is beneficial.
The author thanks Prof. Dr. K.K. Krishnadath gastroenterologist, Academic Medical Centre Amsterdam, known expert in the field of Barrett’s, for her critical comments.
Download Provisional PDF Here
Article Type: Research Article
Citation: Loffeld RJLF (2016) Oesophageal Cancer the First Presentation of Complicated Metaplasia: A Study in Consecutive Patients. J Gastric Disord Ther 2(3): doi http://dx.doi.org/10.16966/2381-8689.120
Copyright: © 2016 Loffeld RJLF. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access