Figure 1: Tilapia nilotica from a private pond in the Yéné district of Franceville. a) Fresh Tilapia nilotica from pond, ready for the experiment. b) Measurement of the mass of Tilapia nilotica.


Ulrich Gauthier Mayombo Mouele1 Christiane Atteke Nkoulembene1 Bertrand Kokolo Ngoma2* Silas Lendzele Sevidzem2 Ibrahim1
1Laboratory of Animal Physiology: Electrophysiology - Pharmacology, Agrobiology Research Unit, University of Sciences and Techniques of Masuku (USTM), Franceville, Department of Biology, University of Science and Technology of Masuku (USTM), Franceville, Gabon*Corresponding author: Bertrand Kokolo Ngoma, Institut de Recherche en Ecologie Tropicale (IRET) / CENAREST, BP.13354, Libreville, Tel: 00241 066 51 82 23/077 81 82 43; E-mail: bertrandkokolo@yahoo.fr
Background and objectives: For nutritional needs, indigenous peasants in Gabon use certain strategies or practices to capture fish. As a result, they use certain parts of ichthyotoxic plants which have biological properties that are toxic for fishes.
Following an ethnobotanical study with a target population made up of men (30%) and women (70%) of all ages, four (4) plants were selected for this study because they were the most cited during our survey, these are: Cylicodiscus gabunensis Harms (26%) (Legume), Tetrapleura tetraptera (Schum. & Thonn.) Taub. (15%) (Fabaceae-Mimosoidae), Zanthoxylum hertzii (Aubrév. & Pellegr.) P.G.Waterman (15%) (Rutaceae) and Brenania brieyi (De Wild) E.M.A. Petit (13%) (Rubiaceae).
Materials and Methods: Biological tests were conducted in order to highlight the ichthyotoxic effect of the selected plants on fish (Tilapia nilotica). The fishes were immersed in 10L of water from the pond contained in a container to which different plant leaf extracts (12.50g, i.e. a dose of 1.25g/L in the immersion medium) were added and rotenone (10-5M) used as a reference molecule (known as an ichthyotoxic substance) and each test was repeated two times. Toxicity was considered or reached when the 1st fish was collected from the surface of the immersion medium with the hand without difficulty, and this was monitored until the last fish (10th).
Results: The current result indicated that in reference immersion medium (pond water) without leaf extracts or rotenone, fish (Tilapia nilotica) were still alive after 60’00, and no loss observed. By comparing the toxicity thresholds of the four-leaf extracts involved in this study and rotenone solutions (10-5M), the boiled Brenania brieyi extract had lowest toxicity threshold (4’19), followed by that of rotenone (10-5M) (8’42). The order of durations of toxic effects of the four-leaf extracts was as follows: For non-boiled extracts, Cylicodiscus gabunensis had the shortest duration of toxicity effect, namely: 2’39, followed by Tetrapleura tetraptera (3’19), Brenania brieyi (3’46), and Zanthoxylum heitzii extract (5’10). Concerning boiled extracts, Tetrapleura tetraptera had the shortest duration of effect, namely: 3’02, followed by Zanthoxylum heitzii (3’10), Brenania brieyi (4’20) and Cylicodiscus gabunensis extract (6’26). For the solutions of rotenone (10-5M), the durations of the toxic effects were 1’57 and 2’08 for unboiled and boiled respectively. Thus, the durations of the toxic effects of the unboiled and boiled solutions of rotenone had the shortest duration of effect compared to those of the leaf extracts involved in this study. Statistical tests revealed significant differences at the 5% level (p<0.05). However, no significant differences were observed between the toxic effects of unboiled rotenone and unboiled Brenania brieyi (Wilcox. Test, p-value=0.1121).
Conclusion: The four unboiled extracts and rotenone solutions (for 60’00) retained their toxicity depending on the weight of the fish, meaning the active ingredients in the plants remained functional even after boiling to cause toxicity. This method of fishing using ichthyotoxic plants can cause undesirable effects to the organism of fish consumers.
Ichtyotoxic plants; Tilapia nilotica; Toxicity; Rotenones; Cylicodiscus gabunensis; Tetrapleura tetraptera; Zanthoxylum hertzii; Brenania brieyi
Among African populations, knowledge on medicinal plants is transferred orally from one generation to the next, but today, this knowledge tends to decline due to the emergence of the society towards modern medicine [1]. Knowledge on the use of ichthyotoxic plants, which is an ancestral technique in Gabon and everywhere [2- 4], facilitates the catching of fish intended for consumption.
In order to identify all ichthyotoxic plants in Gabon, an ethnobotanical survey was carried out across seven (7) provinces of the country. This survey collected useful information from people with knowledge on frequently used ichthyotoxic plant species such as Cylicodiscus gabunensis Harms, Tetrapleura tetraptera (Schum. & Thonn.) Taub., Zanthoxylum hertzii (Aubrév. & Pellegr.) P.G.Waterman. and Brenania brieyi (De Wild.) E.M.A. Petit [5]. The names of all plants have been verified at http://www.theplantlist.org.
According to Cylicodiscus gabunensis, a Guinea-Congolese species is confined almost exclusively to the upper and lower Guinean subcentres [6]. This leguminous plant with high socio-economic value, considered a favorite tree for some people, which is used by rural communities for several purposes [7].
Tetrapleura tetraptera, a plant of the Fabaceae-Mimosoideae family, is generally distributed in the lowland forests of tropical Africa, particularly in West, Central and East Africa [8]. It is widely used by some village communities for the treatment of illnesses such as hypertension, convulsions, leprosy, rheumatic pain, diabetes, and arthritis [9,10]. It is also known for its ichthyocidal effects in certain Africans [11,12].
Zanthoxylum heitzii belongs to the family Rutaceae, and is found in tropical and subtropical regions around the world [13]. In Central Africa, this plant is used for the treatment of many diseases such as malaria, urogenital diseases and syphilis [14]. The work revealed that it is used as poison for fishing in rivers [12,15]. Phytochemical studies indicate that it contains chemicals such as alkaloids, phenols, saponins and terpenoids [16-18].
Brenania brieyi is a perennial flowering plant belonging to the Rubiaceae family. It is present in Nigeria, Gabon, Cameroon and the Central African Republic [19]. This plant is used for the treatment of fever, pain, impotence and infection [20]. The work reported that the sterm bark of Brenania brieyi had anti-inflammatory activity [19]. These pharmacological effects are linked to the presence of certain chemical substances such as flavonoids, tannins, alkaloids, anthraquinones, oses and saponosides [21]. The use of this plant species as a fish poison is well known by Africans [1,11,12].
Regarding biological tests, the work conducted in Gabon [22], those carried out in Nigeria [4,23], revealed that ichthyotoxic plant extracts had effects on Tilapia niloticus (Cichlidae). This fish was reported as one of the first victims of the use of toxic products in fishing [24].
For this traditional fishing practice, the procedure is presented as follows: the various plant parts (stems, bark, seeds, leaves, fruits and herbaceous plants) are crushed and thrown directly into the river. It should be noted that crushed plant extracts are poured into water and mixed with mud or sand for burrowing fish such as catfish, crabs and shrimps [22,25]. The fish after being stunned by the plant extracts are paralysed. The paralysis affects their respiratory systems, thus facilitating their capture at the surface of the water [2]. The active ingredient in the plant extract that causes the paralysis in fish is rotenone [22,26]. Indeed, rotenone molecules, found naturally in plants of the Leguminosae family, have an inhibitory power on the respiratory chain of fish and their target site are gills.
Furthermore, the consumption of fish caught with plant extracts is not recommended for pregnant women [27]. The work reported that local people in Gabon believe that eating this type of fish causes stomach upset [22]. In fact, the fish could still contain in their organs chemical substances which could be the cause of the intoxications and the undesirable effects in consumers. These authors also affirmed that even after having been boiled, the solutions of the leaf extracts could still contain more chemicals to poison fish.
This current study attempts to demonstrate the toxicity of four ichthyotoxic plants of different families namely: Cylicodiscus gabunensis (Legume), Tetrapleura tetraptera (Fabaceae-Mimosoidae), Zanthoxylum hertzii (Rutaceae) and Brenania brieyi (Rubiaceae) which are plant species that are widely exploited by peasants and indigenous Gabonese to capture fish for consumption. Here, biological tests will be conducted on Tilapia nilotica, to assess the effects and toxicity of these ichthyotoxic plants. The objectives of this study are: 1) to verify if there is direct correlation between mass and duration of survival of the fish against substances contained in the ichthyotoxic plants, and 2) to determine the plant whose toxicity is highest in comparison with the rotenone solution whose ichthyotoxic effect is already known.
The biological tests were carried out in the province of Haut-Ogooué, in the Yéné district of the 4th district of Franceville. The fish (Tilapia niloticus) were collected from a pond in the district (Coordinates S 01. 63159°; E 013. 58165°).
The mass of Tilapia nilotica (Figures 1a and 1b) used in this study varied between 25 and 160g. They were collected in their fresh state from the pond and subjected to biological tests.

Figure 1: Tilapia nilotica from a private pond in the Yéné district of Franceville. a) Fresh Tilapia nilotica from pond, ready for the experiment. b) Measurement of the mass of Tilapia nilotica.
Plant samples were collected from 7 provinces of Gabon and were subjected to experiments. These are extracts from the sterm bark of Cylicodiscus gabunensis, Brenania brieyi, Zanthoxylum hertzii and fruits of Tetrapleura tetraptera. These plant parts were first dried in light shade for two weeks and then transformed into powder by referring to the study [28].
The plants were identified at the Institut des Recherchesen Ecologie Tropicale by Botanist Boulanga and the identification was confirmed at the Department of Biology of the University of Science and Technology of Masuku by the botanists of the said Department.
The fish used for this experiment were distributed in containers containing the immersion medium composed of water from the pond (control or reference), at pH=5.62 with temperature varying between 26 and 28°C onto which was added a plant extract of the four different plants (or species) or a solution of rotenone (10-5M) (Sigma) during the various biological tests. Thus, the container contained (experimental environment) 10 L of water from the pond (experimental biotope) and 12.50 g of each plant extract in powder form (i.e.dose of 1.25g/l) or rotenone (10-5M). After homogenization of the medium, 10 fish (Tilapia nilotica) of variable or different masses (g) were introduced and the tests for each ground material of the plant species and solutions of rotenones were repeated 2 (two) times, i.e. 20 fish per extract or rotenone. After the 10 fishes were immersed in the experimental medium, the stopwatch was started (T0), then the time for toxicity effect was recorded when the 1st fish was collected on the surface of the medium of the container with the hand without difficulty, and this was monitored until the last fish (10th). Overall, this protocol led to the evaluation of the effect of the dose (1.25g/l) of the plant extract or rotenone (10-5M) as a function of time and the mass of the fish. The tests are repeated two times for each plant extract or rotenone (10-5M).
To compare the reaction time of each plant and that of rotenone (rotenone (l0-6M) taken as a control) statistical tests (Friedman test; Wilcoxon test) were applied from the software R.3.4.3. In case of multiple comparisons, P-values were adjusted by the Holm method.
For each plant extract and rotenone, the time taken to intoxicate the fish varied significantly between treatments: plant extract and rotenone not boiled (Friedman’s test; statistic=38, 211, df=4, p-value=0.00001014), plant extract and rotenone boiled (Friedman’s test; statistic=33.04, df=4, p-value=0.000001172).
No effect of the immersion protocol was detected. The results show that no loss of Tilapia nilotica (20) was observed after 60’00 in the immersion medium (pond water) without leaf extracts or rotenone Figure 2, and during this period all experimental fishes remained alive.
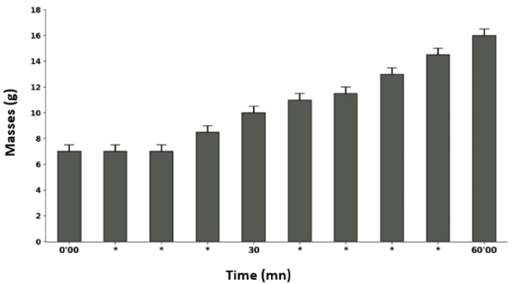
Figure 2: Effect of immersion medium (pond water) without plant extracts or rotenone on Tilapia nilotica in vivo after 60’00.
Non-boiled Cylicodiscus gabunensis leaf extracts had the lowest toxicity threshold (9’00) compared to its boiled (10’49) counterpart and the maximum effect was 11’39 and 17’ respectively. That is successively for a duration of toxicity of 2’39 and 6’26 (Wilcox. test, W=5, p-value=0.000765; Figure 3).
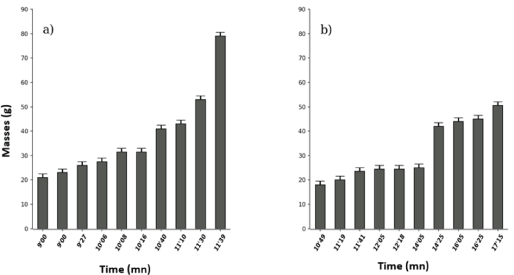
Figure 3: Effects of non-boiled (a) and boiled (b) Cylicodiscus gabunensis (Legume) on Tilapia nilotica in vivo.
Boiled Brenania brieyi leaf extract had the lowest toxicity threshold (4’19) compared to non-boiled (7’06) and the maximum effect is 8’39 and 10’ respectively. That is successively for a duration of toxicity of 4’20 and 7’46 (Wilcox. test, W=88.5, p-value=0.004058, figure 4).
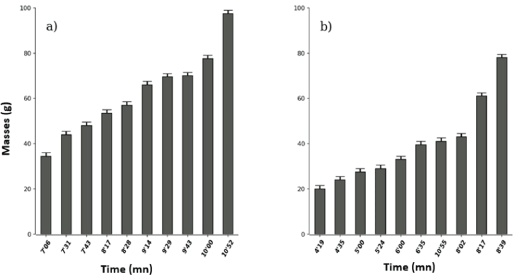
Figure 4: Effects of unboiled (a) and boiled (b) Brenania brieyi (Rubiaceae) on Tilapia nilotica in vivo.
Non-boiled Tetrapleura tetraptera leaf extracts had the lowest toxicity threshold (6’10) compared to boiled (8’39) and the maximum effect was 9’12 and 11’ respectively. That is successively for duration of toxicity of 3’02 and 2’58 (Wilcox. Test, W=5, p-value=0.000765, figure 5).
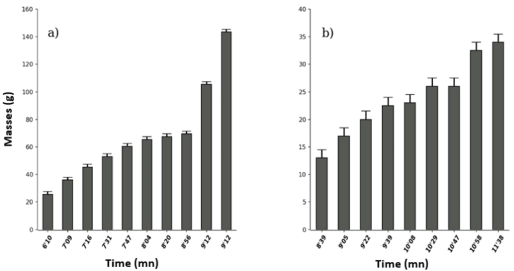
Figure 5: Effects of unboiled (a) and boiled (b) Tetrapleura tetraptera (Fabaceae) on Tilapia nilotica in vivo.
Figure 6 shows that non-boiled Zanthoxylum heitzii leaf extract had the lowest toxicity threshold (6’15) compared to that of its boiled (7’50) counterpart and the maximum effect was 9 ‘25 and 13’06 respectively, i.e. successively for a duration of toxicity of 3’10 and 5’16. The statistical test revealed a significant difference (Wilcox. Test, W=11, p-value=0.002089).
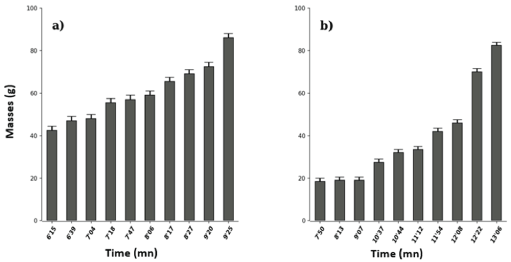
Figure 6: Effects of unboiled (a) and boiled (b) Zanthoxylum heitzii (Rutaceae) in vivo on Tilapia nilotica in vivo.
Figure 7 shows that boiled rotenone solution (10-5M) had the lowest toxicity threshold (8’42) (Figure 6b) compared to the nonboiled one (27’47) (Figure 6a) and the maximum effect was 10’50 and 29’44 respectively (Wilcox. Test, W = 100, p-value = 0.0001817, Figure 6). That is successively for duration of toxicity of 2’08 and 2’00.
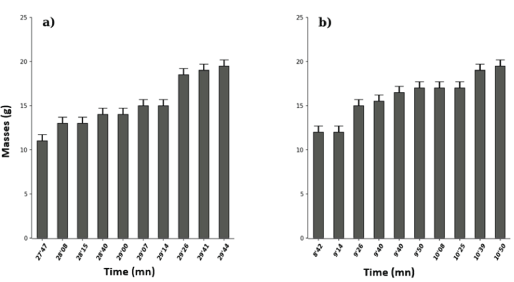
Figure 7: Effects of rotenone (10-5M) unboiled (a) and boiled (b) on Tilapia nilotica in vivo.
Faced with certain circumstances of life, Man is able to adopt and integrate behaviors or practices inorder to survive. Thus, to have fish for its nutrition, Man has developed certain practices or methods (strategies) to capture fish intended for consumption, and this is the case of the use of ichthyotoxic plants.
From our study, it appears that in fish immersion medium without leaf extracts or rotenone, which served as a control test (pond water), no loss was observed. The 20 fishes (Tilapia nilotica) remained alive after 60’00. This result is similar to those who reported that there was no loss of Clarias gariepinus after 96 hrs of immersion in a medium without leaf extracts [4]. Similar observations have already been reported on juvenile Oreochromis niloticus (Cichlidae) [24,29]. This study is the first observation of the toxicity tests of four ichthyotoxic plants exploited by the Gabonese populations for fishing in rivers and lakes.
The effects of four (4) leaf extracts (Cylicodiscus gabunensis, Brenania brieyi, Tetrapleura tetraptera and Zanthoxylum heitzii), unboiled and boiled rotenone had toxic effect on fish (Tilapia nilotica In vivo) and this toxicity was mass dependent, where the smallest fish were first intoxicated and caught easily with the hand at the surface of the water from the pond contained in the container.
By comparing the toxicity thresholds of the four (4) leaf extracts involved in this study and rotenone dose (10-5M), the boiled Brenania brieyi extract had the lowest toxicity threshold (4’19), followed by that of boiled (8’42) rotenone (10-5M). Regarding the order of the durations of the effects of the 4 leaf extracts: For non-boiled extracts, Cylicodiscus gabunensis had the shortest duration of effect, followed by Tetrapleura tetraptera, Brenania brieyi, and extract of Zanthoxylum heitzii (5’10). For boiled extracts, Tetrapleura tetraptera had the shortest duration of effect, followed by Zanthoxylum heitzii, Brenania brieyi (4’20) and the extract of Cylicodiscus gabunensis (6’26). For the solution of rotenone (10-5M), the durations of the effects were 1’57 and 2’08 for non boiled and boiled respectively. Thus, the durations of the effects of the unboiled and boiled solutions of rotenone are the shortest compared to those of the leaf extracts involved in this study.
Indeed, the investigations revealed that certain plants were toxic to fishes [30]. Studies showed that Tephrosia vogelii from the Fabaceae family and Justicia extensa from the Acanthaceae family intoxicated Tilapia nilotica [22]. The study showed that the leaves of Tephrosia vogelii were toxic to juvenile of Oreochromis niloticus [24]. Study on the impact of humans on ecosystems by using ichthyotoxic plants to capture fish for food, found that T. vogelii, A. cissampeloides and A. vogeliana were toxic to Clarias gariepinus (Catfish) [3]. Studies revealed that T. vogelii, A. cissampeloides and A. vogeliana poisoned the same animal species (Clarias gariepinus (Catfish)) [4].
From the results of the current study, we found that, even after boiling the leaf extracts and the solutions of rotenones for 60’00 (time of one boiling) to “destroy” or “neutralize” the substances contained in their composition, the toxicity persists at the level of the four (4) species and the solutions of rotenones, because they still intoxicate the fish, which can cause undesirable effects to consumers of fish from this type of fishing practice, because the latter could contain in their organs molecules from the extracts that made it easy to capture them.
Indeed, some studies had reported that ichthyotoxic plants could cause undesirable effects to consumers of fish from this fishing technique [11,22,31] revealed that this type of fishing method represent danger for consumers of fish because the consumption of fish caught using this approach could cause diarrhea as highlighted in the report [3,32]. Contrary, a study reported that even after consuming intoxicated fish, consumers did not experience any undesirable disorders [33]. From literature it is known that the leaf extracts of Cylicodiscus gabunensis, Brenania brieyi, Tetrapleura tetraptera and Zanthoxylum heitzii currently studies contain secondary metabolites for their biological and pharmacological properties including: tannins, steroids/terpenes, saponins, alkaloids, polyphenols, flavonoids, reducing sugars, glycosides and anthocyanins [12]. Thus, these chemical compounds of the four (4) ichthyotoxic plants could be involved in the poisoning of Tilapia nilotica.
Indeed, the study revealed that Justicia secunda Vahl (ichthyotoxic plant) had secondary metabolites such as: phenols, flavonoids, alkaloids, tannins, glycosides, saponins, coumarins and terpenes, which could be involved in the biological properties of this plant (Justicia secunda). Derris elleptica and Tephrosia vogelii (ichthyotoxic plants) contained retinoids (one of the chemical components of these plants) that are used as insecticides and probable anti-carcinogenic [34]. The study showed that the powder of the roots of Tephrosia Virginiana (Fabaceae) can be used as a pesticide, because this ichthyotoxic plant contains certain molecules such as rotenone that cause its biological effect [35]. The study highlighted the use of indigenous plants with an insecticidal effect [36] and showed the antimicrobial activity of the aqueous extract of Eryngium foetidium [37]. The study revealed that ichthyotoxic plants, such as Justicia secunda Vahl, contain in their composition, secondary metabolites which have pharmacological properties (phenols, flavonoids, alkaloids, tannins, glycosides, saponins, coumarins, terpenes) capable of treating certain human diseases [34]. The study revealed the respiratory disorders caused by saponin in fish [38,39]. Also, the study underlined the toxic effect of alkaloids on fish [40]. Furthermore, the study reported that Maesa lanceolata, a saponin plant was used to intoxicate and capture fish [41]. For solutions of synthetic rotenone (10-5M) used as reference molecule (known for its ichthyotoxic effect), the toxic effects duration were 1’57 and 2’08 for the unboiled and boiled solutions respectively. Indeed, the studies are similar to these results that Tephrosia vogelii (a rotenone plant) has a property that asphyxiates Clarias gariepinus (Catfish) by intoxicating them (toxicity) [3]. The study showed that ichthyotoxic plants of the Leguminosae family with rotenone and retonoids contained in their chemical composition, cause paralysis of fish [42].
After an ethnobotanical survey previously conducted in 7 provinces of Gabon, information was collected on the use of ichthyotoxic plants, and then the chemical families contained in these plants were determined by phytochemical screening. From there, four (4) of the most used plants were used for biological tests on Tilapia nilotica in vivo in order to confirm or deny the information collected from the surveyed populations.
Indeed, all four plants extract intoxicated fish (Tilapia nilotica), this intoxication was mass dependent, since small fish were the first to be intoxicated at a dose of 1.25g/L for each extract studied. The time that characterizes the threshold of toxicity varied according to the plant extract, not carried and carried to boiling. The observed toxicity is a function of the mass of the fish whatever the species.
Thus, the durations of the effects of solutions of unboiled and boiled rotenone (10-5M) were shortest compared to those of the leaf extracts involved in this study. The synthetic unboiled and boiled rotenone leaf extracts of Cylicodiscus gabunensis, Brenania brieyi, Tetrapleura tetraptera and Zanthoxylum heitzii were toxic to Tilapia nilotica.
In sum, the four (4) extracts and solutions of rotenone’s not carried and carried to boiling (during 60’00) keep their toxicity according to the mass of fish, which means that the active principles that compose the plants remain functional even after carrying them to boiling to cause toxicity (or their toxic effect), which can cause undesirable facts to the organism of the consumers of the fishes resulting from this method of fishing using the ichthyotoxic plants.
Authors are grateful to Mr. Gabriel Bounda for accepting to provide his ponds for this experiment. Authors are thankful to the technicians of the Department of Biology for assisting in experiments carried out at the Agrobiology laboratory of the University of Sciences and Technique of Masuku.
There is no conflict of interest.
Mr. Ulrich Gauthier Mayombo Mouele and Dr. Bertrand Kokolo collected the data in the field and participated in the writing of the manuscript. Dr. Bertrand Kokolo and Dr. Silas Lendzele Sevidzem performed the data analysis. These data were verified by Prof. Christiane Atteke Nkoulembene and Prof. Ibrahim. Silas Lendzele Sevidzem and Mr. Ulrich Gauthier Mayombo Mouele translated the manuscript into English.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Mouele UGM, Nkoulembene CA, Ngoma BK, Sevidzem SL, Ibrahim (2022) An In vivo Test on the Ichthyotoxic Properties of Four Plants (Cylicodiscus gabunensis Harms, Brenania brieyi (De Wild.) E.M.A. Petit, Tetrapleura tetraptera (Schum. & Thonn.) Taub. and Zanthoxylum heitzii (Aubrév. & Pellegr.) P.G. Waterman.) Used By Indigenous Farmers on Tilapia nilotica in Gabon. J Envi Toxicol Stud 4(2): dx.doi.org/10.16966/2576-6430.133
Copyright: © 2022 Ngoma BK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access