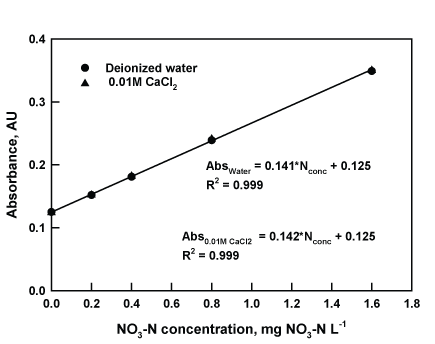
Figure 1: Calibration curve of NO3-N standards (0-1.6 mg NO3-N L-1) prepared in deionized water and CaCl2 matrices.


Iseyemi O1 Adviento-Borbe MAA1* Haas L2 Farris JL2 Reba ML1 Massey JH1
1Delta Water Management Research Unit, United States Department of Agriculture-Agricultural Research Unit, USA*Corresponding author: Adviento-Borbe MAA, Delta Water Management Research Unit, United States Department of Agriculture-Agricultural Research Unit, USA; E-mail: arlene.advientoborbe@ars.usda.gov
A single-reagent spectrophotometric procedure using vanadium (III) chloride (VCl3 ) was found to provide accurate and robust measurement of low levels of nitrate (NO3 -N) in agricultural runoff. Results of the VCl3 method produced data that correlated well (r=0.86; p<0.001) with NO3 -N concentrations determined using the standard cadmium NO3 reduction method. For both natural waters and solutions prepared to mimic agricultural runoff, limits of detection and quantitation were 0.0 and 0.04 mg NO3 -N L-1, respectively, while NO3 recoveries ranged from 97 to 100%. The VCl3 method was accurate at low nitrate concentrations (0.03 to 1.6 mg NO3 -N L-1) and required 99% less sample than the standard cadmium method. These results indicate that the vanadium reduction method accurately quantify trace amounts of NO3 -N in terrestrial water samples such as surface water and agricultural runoff.
Nitrate; Water; Vanadium reduction method; Cadmium reduction method
Nitrate (NO3 ) is a prevalent contaminant in freshwater systems, with important agricultural and human health consequences [1]. It is also an essential nutrient and critical for optimum crop production [2]. In agriculture, about 50% of applied inorganic nitrogen fertilizer is used for plant growth, while the remainder is lost as NO3 into freshwater via combination of leaching (25%) and surface runoff (5%) and as gaseous forms like nitrous oxide (N2O) through microbial denitrification/nitrification and ammonia (NH3 ) via volatilization (20%) [2,3]. The presence of high levels of NO3 , together with phosphorus (P) in waterways, can enhance eutrophication and lead to harmful algal blooms and oxygen depletion in downstream ecosystems [4,5]. Elevated NO3 levels in drinking water sources are a continuing human health concern because of potential contributions to methemoglobinemia or blue baby syndrome (reduction in infant blood oxygen carrying capacity) and gastric cancer risks from NO3 contamination [1]. Recognizing the influence of nitrogen (N) application on crop yields, contamination offsite, and human health effects of long term NO3 exposure, it is important to account for NO3 ions in the environment using an accurate, robust, and reliable quantification procedure.
A number of procedures for the detection and determination of NO3 in water and other matrices have been employed and reported elsewhere. These include capillary electrophoresis [6], flow injection analysis/automation and electrochemical detection [7], chromatography with fluorescence detection [8,9], chemiluminescence [10], and various spectrophotometric techniques [1,11]. While the chemistry underlying most of these methods is explicitly discussed and adopted, many of the methods are not sensitive in detecting lower concentrations of NO3 (<10 mg L-1) and also suffer from compound interferences [12]. Spectrophotometric methods are commonly used due to their simplicity of procedure, wide detection range, and minimum requirement for specialized or expensive equipment [11,13]. Recent studies have shown a spectrophotometric procedure of NO3 reduction by vanadium (III) chloride (VCl3 ) compared to reduction by cadmium was reliable, cost effective, safe, and convenient for NO3 determination in water and soil extracts [11,14]. Vanadium (III) in acid solution is used to reduce NO3 to nitrite (NO2 ). As NO2 is formed, it is captured by Griess reagents (N-(1-Naphthyl) ethylenediamine dihydrochloride (NEDD), sulfanilamide and phosphoric acid). The method consists of all reagent chemicals combined into one solution and requires a small volume of aliquot to develop color for spectrophotometric measurement. The vanadium reduction method provides reliable data replication with detection of NO3 at low concentrations (in the range of µg L-1), as opposed to cadmium reduction, which may be less sensitive to low NO3 concentrations [11]. In comparison with the cadmium reduction/ Griess reaction, the vanadium reduction method requires less chemicals, convenient and low-cost laboratory equipment and materials, and less sample, while generating less waste [14]. Although procedural steps may be streamlined, additional time might be expected in analyzing hundreds of samples. However, time required for reagent preparation is minimal and reagent, once prepared, can be stored at 4℃ in the dark to avoid oxidation [11] and used as needed over a period of two weeks. Objectives of the current study were to further assess the sensitivity and reliability of the VCl3 procedure for determining NO3 -N concentrations in water and salt solution matrices, and compare these results to those obtained using the cadmium reduction method.
Sulfanilamide, NEDD, VCl3 , sodium NO2 , sodium nitrate, calcium chloride, and hydrochloric acid, were all purchased from either SigmaAldrich or Thermo Fisher Scientific. All chemicals were reagent plus grade (assay >99%) and were used without further purification. Vanadium (III) chloride tends to generate corrosive fumes, especially when exposed to moist air. Hence, absorption of water vapor into the VCl3 bottle was minimized by storing in the dark and keeping the opened bottle inside a sealed vacuum desiccator with ample amount of anhydrous calcium sulfate desiccant.
To prepare the solution, 0.5 g VCl3 were added to 200 mL 0.5 M HCl. Oxidation of VCl3 was reduced by weighing VCl3 directly into the flask with 200 mL 0.5 M HCl in a hood. The solution was briefly shaken to dissolve VCl3 , and any un-dissolved particles were filtered through a 0.45µm pore size cellulose acetate syringe filter. Since VCl3 is not classified as harmful to the environment or toxic (supplier SDS data), there were no excessive precautions needed in preparing the reagent. Next, 0.2 g sulfanilamide and 0.01g NEDD were added to the VCl3 solution. This reagent is stable for up to two weeks if stored at 4℃ [11,14].
For water samples with levels ranging from 0.3 to 10 mg NO3 -N L-1, 100 µL sample aliquots and 800 µL VCl3 reagents were transferred and mixed directly in 3-mL semi-micro cuvettes (Brandtech Scientific Inc., CT, USA). For concentrations >10 mg NO3 -N L-1, a 1 mL sample aliquot was diluted five to 10 times (depending on the concentration) prior to addition of VCl3 reagent. Immediately after chemical reagent addition, indophenol blue color was developed at room temperature (20-25℃). The color was stable for about 48 h. Absorbance measurements were made at 540 nm using a UV/VIS Model 1800 spectrophotometer (Shimadzu, Inc., Japan). Since VCl3 method is based on reduction of nitrate to nitrite, the presence of nitrite in water samples was also determined by analyzing the same water samples using the same method and reagent but without vanadium in the mixture. Hence, to determine the nitrate levels in the water samples, the nitrite measured without vanadium reagent was subtracted from the nitrite analyzed by reagent with vanadium (III) chloride.
A 25 mL sample aliquot, a HACH®DR/890 colorimeter (Loveland, CO, USA) and HACH colorimeter nutrient commercial standard reagent pillows were used. A NitraVer 6 NO3 reagent powder pillow was added to 15 mL of sample in a sample cell, and the mixture was shaken vigorously for 3 minutes. Cadmium metal in the pillow reduced NO3 present in the sample to NO2 . After 2 min of complete reaction, 10 mL of the resultant solution was transferred to an empty sample cell and the contents of a NitriVer 3 NO2 reagent powder pillow were added. Nitrite ion reacts in an acidic medium with sulfanilic acid to form an intermediate diazonium salt which combines with chromotropic acid to form a pink-colored product. The pink color development was maximum after 15 min and absorbance reading was measured at 520 nm using a HACH®DR/890 colorimeter (Loveland, CO, USA). The remaining 10 mL sample aliquot from the original 25 mL sample was used as the blank sample. Blank samples were measured at every color measurement of sample.
To establish limits of detection and determination for the VCl3 method, blanks with various matrices (deionized water and salt solutions were utilized). Using a sample to reagent ratio of 1:8, 100 µL of water sample aliquot or 100 µL aliquot of 0.1 M CaCl2 solution and 800 µL of VCl3 reagent were transferred and mixed in a 3-mL semi-microcuvette. For each matrix (water or CaCl2 solution), 10 blank samples were prepared and allowed to stand for 8 h at 25℃ for maximum color development. Next, absorbance was measured at 540 nm using a UV/VIS 1800 spectrophotometer (Shimadzu, Inc, Japan).
Limit of detection, LD, defined as the lowest analyte concentration reliably distinguished from analytical noise (signal produced in the absence of NO3 ), was estimated using Equation 1 as described by Currie [15].
\[{L_D} = 4.65 \times {\partial _B}\,\,\,\,\,\,\,\,\,\,\,\,[1]\]
Where∂B is standard deviation of NO3 concentrations (mg NO3 -N L-1) of 10 blank samples. Limit of determination (quantitation), LQ, defined as the lowest analyte concentration reliably distinguished and yielding a satisfactory quantitative estimate was used with a statistical level of confidence set at 95%, standard deviation of 10% and random errors being normally distributed. The LQ of vanadium reduction method was estimated using Equation 2 as described by Currie [15].
\[{L_D} = 14.1 \times {\partial _B}\,\,\,\,\,\,\,\,\,\,\,\,[2]\]
Nitrate-N standard curves were prepared in two matrices; namely deionized water and 0.01M CaCl2 . The 0.01 M CaCl2 was chosen in addition to deionized water because CaCl2 -solution mimics the presence of salts in soil solution concentrations and irrigation water [16,17]. Calcium is a major cation available in soil adsorption complex and exchange processes that can easily be desorbed in soil water. Absorbance values of standards prepared with 0.01 M CaCl2 matrix were compared to standards prepared in deionized water to assess any matrix interference with maximum color development. A 1,000 mg NO3 -N L-1 stock solution was prepared by dissolving 1.37 g of NaNO3 in 1 L of deionized water. A low standard range (0 mg NO3 -N L-1, 0.2 mg NO3 -N L-1, 0.4 mg NO3 -N L-1, and 1.6 mg NO3 -N L-1) and a sample to reagent ratio of 1:8 were used in this study, as recommended by Doane and Horwath (2003) [14]. Nitrate-N standard concentrations were analyzed using the vanadium reduction method. Ten replicates of standards were prepared at three separate times and solution absorbance was measured at room temperature (25℃) after approximately 8 h, within which maximum color development was reached. Zero absorbance was set using the corresponding blanks for both matrices.
Three sample matrices with NO3 -N concentrations between 0.1 and 0.4 mg NO3 -N L-1 were spiked with known amounts of NO3 -N and prepared to assess potential interference of any matrix constituents on NO3 estimation, as well as to ascertain the procedure reproducibility. To 49.9 mL of each of the two sample matrices, 0.1 ml of 100 mg NO3 -N L-1 (prepared from standard stock solution) was added. Three replicates were prepared for both original samples and spiked samples. Nitrate-N concentrations were analyzed using the vanadium reduction method and NO3 -N levels in all samples were determined using calibration N standards prepared in deionized water. Percent recovery of the added mg NO3 -N L-1 was estimated as described in Equation 3 [18].
\[\% R = \frac{{(spiked{\rm{ }}sample{\rm{ }}concentration - unspiked{\rm{ }}concentration)}}{{known{\rm{ }}spike{\rm{ }}added{\rm{ }}concentration}} \times 100\,\,\,\,\,\,\,\,\,\,\,[3]\]
A set of 52 runoff water samples were collected from a commercial row crop agricultural production farm (CLRF) located in Craighead County, AR, and two watersheds in the vicinity of the farm located in Little River Ditches Basin (LRDB), Mississippi County, and Lower St. Francis Basin (LSFB), Poinsett County, AR with total area of 461 ha, 5,340 ha, and 2,335 ha, respectively. Associated land area was planted in cotton, rice, and soybeans. In the CLRF site, grab water samples were collected from two ditches draining the production area during the growing season between 25 May 2016 and 25 August 2016. Ditches served as feeder canals supplying water to a tail water recovery irrigation system. In both LRDB and LSFB sites, each had five in stream water quality monitoring stations where water samples were collected weekly from 6 April 2017 to 23 May 2017. Water samples were collected during early crop growing season to obtain different ranges of NO3 -N levels in the field. Samples were from discharge events involving precipitation, irrigation, and irrigated field draw down and immediately stored on ice for transport to the laboratory. Water samples were filtered with 0.45-µm cellulose acetate syringe filters within 24 h prior to chemical analyses. Water samples were analyzed for NO3 -N by VCl3 reduction procedure as described above at the Water Quality Research Laboratory, Delta Water Management Research Unit (DWMRU), United States Department of Agriculture (USDA)-Agricultural Research Services (ARS), Jonesboro, AR, and the cadmium reduction standard method [19] at the Aquatic Ecology Laboratory, Arkansas State University. To avoid temporal changes of NO3 -N concentrations in water samples over time, water samples were analyzed using both methods within 24 hours of sampling.
Data were checked for homogeneity of variance using Levene’s test (for any continuous distribution), while normality assumptions (samples normal distribution) were checked using the Shapiro-Wilk test. When datasets failed the normality test, data were log transformed to validate the parametric statistical assumption. Two sample t-tests were performed for each level of standard concentration to assess differences in the absorbance readings between standard values of deionized water matrix and 0.01M CaCl2 matrix. Pearson linear correlation and linear regression was used to assess any relationship between NO3 -N concentrations obtained in water samples analyzed using the cadmium reduction method and the VCl3 chloride reduction procedure. Significant level for alpha was set to 0.05 for all tests. All statistical analyses were performed using Minitab® Statistical Software ver. 17 (Minitab Inc., U.S.A.).
Limits of detection and determination for VCl3 reduction method
Differences in absorbance readings of blanks ranged from 0.124 to 0.350 nm and percent coefficient of variation was 19.0% for both matrices. The percent of variation calculated in all matrices indicated any small and similar absorbance noise signal that may have occurred during color measurements across replicates and matrices. Limit of detection (LD) was 0.01 mg NO3 -N L-1 for both deionized water and 0.01 M CaCl2 matrices, while limits of determination were 0.03 mg NO3 -N L-1 and 0.04 mg NO3 -N L-1, respectively. A similar LQ estimate (0.03 mg NO3 -N L-1) for marine and freshwater samples was reported by Doane and Horwath (2003) [14] while LQ of 0.02 mg NO3 -N L-1 was reported by Woollard et al. [20] in nitrate analysis of milk and milk powder. In addition, Schnetget et al. [21] found also a limit of detection of 0.01 mg NO3 -N L-1 for the analysis of terrestrial water samples. In the studies conducted by Wang et al. [22] and Garcia Robledo et al. [23], they reported a much lower LD estimate of 0.001 mg NO3 -N L-1 using an automated flow injection analyzer and spectrophotometer with thermostatized cuvette holder regulated by an external water bath for nitrate analysis of freshwater and marine water samples, respectively. Apparently, the lower detection limits reported by these studies than our study were due to differences in reaction time, temperature and aqueous media to which NO3 -N was measured. The detection limit in our study was the same as reported for the cadmium reduction method (LD=0.01 mg NO3 -N L-1) using the above HACH instrument. For the VCl3 reduction method, NO3 -N concentrations of 0.03 and 0.04 mg NO3 -N L-1 (LQ) were the lowest NO3 -N levels that can be estimated in water and CaCl2 matrices, respectively and had the lowest risk of committing a false measurement. The LQ thresholds estimated in this study were about 300 times lower than the critical standard of 10 mg NO3 -N L-1 for drinking water [24]. Our findings indicate the VCl3 reduction method is capable of measuring NO3 -N concentrations in water and CaCl2 samples down to trace concentrations with high precision.
The absorbance of different levels of standards prepared in deionized water was compared with those prepared in CaCl2 solutions to determine interferences of matrix constituents. There were no significant differences between the average absorbance measured in NO3 calibration samples that had deionized water and 0.01M CaCl2 matrices (P-level=0.07-0.87) across all levels of standards. Due to similarity of absorbance readouts, there were identical linear curves derived from series of standards used in both matrices with a coefficient of determination (R2 ) of 0.999 (Figure 1). A linear response with nitrate concentrations ranged from 0 to 40 µM NO3 (2.5 mg NO3 L-1) was also found by Garcia Robledo et al. [23] in their assessment of VCl3 reduction method using nitrate standard samples. Moreover, in this study average percent recovery of spiked NO3 -N in aqueous samples ranged from 96 to 100% across all matrices (Table 1). This range was within the percent recovery range reported by Schnetger et al. [21] and Wang et al. [22] (89 to 108%) when VCl3 reduction method was evaluated using freshwater samples (i.e. lake, river, rain, tap water).

Figure 1: Calibration curve of NO3-N standards (0-1.6 mg NO3-N L-1) prepared in deionized water and CaCl2 matrices.
| Sample to reagent ratio (µL) | Initial NO3 -N (mgL-1) | Recovery of spiked (%) | |
| Deionized Water | 100:800 | 0.2 | 95.7 (2.68) |
| CaCl2 | 100:800 | 0.2 | 96.7 (2.68) |
| Deionized Water | 100:800 | 0.4 | 98.4 (1.22) |
| CaCl2 | 100:800 | 0.4 | 100 (2.68) |
| Tap water | 100:800 | 0.2 | 98.5 (6.00) |
Table 1: Mean percent recovery of aliquot of nitrate added to samples (n = 3). Values in parenthesis are standard errors of the mean measurements.
Our findings illustrate similar and/or minimal to zero matrix interference in all levels of standards for both deionized water and Ca-solution matrices in the analysis of NO3 -N using the VCl3 reduction method. Additionally, interferences of other non-target analytes (CaCl2 solution) were insignificant in the NO3 -N analysis of samples in the presence of increasing NO3 -N concentrations. Since the CaCl2 matrix did not significantly affect absorbance of standards when compared to a water matrix, any of these matrices can be used to prepare sets of standards to analyze water samples, provided the sample to reagent ratio is 1:8 and color development is at maximum. For concentrated samples or having a sample to reagent ratio of <1:8, matrix interferences should be quantified to account for interferences and limits of determination. Garcia Robledo et al. [23] suggested diluting water samples with high nitrate and nitrite concentrations to <40 µM nitrate (2.5 mg NO3 L-1) to provide more accurate estimate of nitrate concentration in water using the VCl3 reduction method. Furthermore, high salt content can interfere in the absorbance response and may lead to underestimation of NO3 -N in water samples. Wang et al. [22] found a decrease in the sensitivity of nitrate analysis using VCl3 reduction method at increasing salt contents (0-80 µM) in water samples. Garcia Robledo et al. [23] reported that salt effect remained stable in salinities between 20 and 50 (32 and 73 mS cm1 ) and had similar absorbance response (94%) of the value obtained in distilled water. Thus, it has been suggested that calibration curves should be prepared in matrices with ranges of salinities found in samples to be analyzed to avoid significant interferences of non-target analytes. Our data strongly support the high reproducibility and high recovery of the VCl3 reduction method for NO3 -N analysis of water and CaCl2 samples as reported by Doane et al. [14].
Water samples from different agricultural fields were analyzed using both VCl3 reduction procedure and cadmium reduction method. Nitrate concentrations determined using a set of standards (0 to 1.6 mg NO3 -N L-1) prepared in deionized water ranged from 0.03 to 1.68 mg NO3 -N L-1. Across all three sites and sampling dates, there were strong linear correlation (r=0.86; P-value <0.0001) and linear fit (R2 =0.73; R=0.85) observed in NO3 concentrations obtained in samples analyzed using the cadmium reduction standard method and the VCl3 reduction procedure (Figure 2). Similarly, studies conducted by Wang et al. [22], Schnetger et al. [21], and Cecchini et al. [25] found a similar fit between the VCl3 reduction and cadmium reduction methods using surface water samples with concentrations below 62 µM nitrate (1 mg NO3 -N L-1). Mean difference between measurement results from cadmium reduction and VCl3 reduction methods was 14.83%, where VCl3 reduction procedure was higher than that of the cadmium reduction standard method. This value is much higher compared to average differences (3.4%) reported by Doane et al. [14] for similar matrices and NO3 methods comparison. In Doane and Horwath study [14], the average differences were calculated from water samples with a higher range of NO3 concentration (1 to 10 mg NO3 -N L-1) and fewer data points for slope estimates (3); whereas in our study, we used 5 data points and water samples had lower concentration range of 0.1 and 1.6 mg NO3 -N L-1. In this study, comparison of cadmium reduction method to VCl3 method was conducted using surface runoff water from 16 water sampling locations that represent wide range of actual water quality characteristics occurring in a commercial field and/or natural watershed. Variability associated with different sources of surface water samples were fully accounted for in the data analysis. This analysis is important in the validation of colorimetric method for routine water testing.
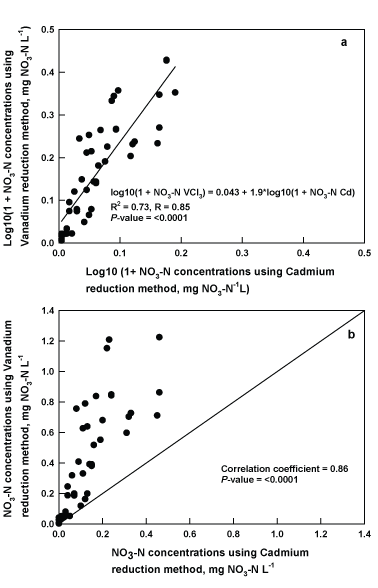
Figure 2: Relationship of nitrate concentrations in water samples from various agricultural fields located in Craighead County, Little River Ditches Basin (LRDB) in Mississippi County, and Lower St. Francis Basin (LSFB) in Poinsett County, AR measured by cadmium reduction method and the vanadium reduction procedure.
It appears that the cadmium reduction method is less sensitive in reducing NO3 ions to NO2 prior to reaction with diazotizing reagent (sulfanilamide) at very low levels of NO3 in water samples. Miranda et al. [11] also observed the reduced sensitivity of the cadmium reduction method to lower NO3 -N concentrations and suggested this method may have under estimated nitrate concentrations in comparison to the VCl3 reduction method. One potential reason for the underestimation of NO3 -N using the cadmium reduction method is presence of dissolved organic C (DOC) in water samples from terrestrial soils and marine sediments that leads to degradation of the reduction cadmium column. Schnetger et al. [21] tested the influence of DOC on 20 µM nitrate calibration solution with spiked DOC (100 mg DOC L-1) and found no interference (<2%) on the efficiency of the reduction. Overall, our use of the VCl3 reduction procedure with water samples provided accurate estimates of NO3 -N at low analyte concentrations.
This study has reported repeatability and reliability of an alternative procedure that involved reduction of NO3 by VCl3 reagent for quantification of NO3 -N concentrations in natural waters and Casolution. The method does not require elaborate reagent preparation time or specialized equipment and is suitable for small volume of sample aliquot (<1 mL). The method uses 99% less water samples as compared to standard cadmium reduction method and more accurately quantifies trace amounts of NO3 -N in waters. The favorable strong correlation with the standard cadmium reduction assay, as well as negligible matrix interferences at low levels, demonstrate the appropriateness of this method for routine measurement of NO3 -N in terrestrial water samples such as surface water. While other studies reported the applicability of VCl3 reagent reduction method in medical, food, and controlled laboratory experiments, this study further demonstrated the applicability of this method for measuring NO3 -N contents in agricultural surface water with varying degree of water quality properties occurring in a commercial field and/ or watershed. Likewise, while other reported studies indicated less sensitivity of cadmium reduction method in quantifying NO3 -N in samples with a higher range of NO3 concentration (1 to 10 mg NO3 -N L-1) when compared with VCl3 reagent reduction method, this study demonstrated a much more reduced sensitivity of the cadmium reduction method compared with VCl3 reagent reduction method in a lower NO3 -N range (0.1 and 1.6 mg NO3 -N -1).The VCl3 method is highly suitable for limited sample volume due to sampling protocol such as soil solution, plant sap or availability of samples during collection such as rain water. This method can also be a useful routine alternative for compliance testing of NO3 and NO2 in medical, food and research laboratories.
We thank K. Kahill for her help in water sampling in various agricultural fields. We also are grateful to the producers who allowed the sample collection on their farms, namely Jerry Don and Tommy Clark, Wildy Family Farms, Steve Craig, and Ritter Agribusiness.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Iseyemi O, Adviento-Borbe MAA, Haas L, Farris JL, Reba ML, et al. (2018) Validation of a Spectrophotometric Procedure for Determining Nitrate in Water Samples. J Environ Toxicol Stud 2(1): dx.doi.org/10.16966/2576-6430.113
Copyright: © 2018 Iseyemi O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access