
Figure 1: Diagram of the main cholesterol metabolic pathways in the liver.


Maâmar Souidi* Radjini Racine
Institute for Radiological Protection and Nuclear Safety. Laboratory of Experimental RadioToxicology. Fontenay-aux-Roses, France*Corresponding author: Maâmar Souidi, Institute for Radiological Protection and Nuclear Safety. Laboratory of Experimental Radio Toxicology. Fontenay-aux-Roses, France, Tel: +33158359194; Fax: +33158358467; E-mail: maamar.souidi@irsn.fr
Cholesterol hepatic metabolism was studied in rats with a focus set on Cytochrome P450 (CYP) gene expression after a chronic ingestion of depleted uranium for 9 months (ca. 20 years for humans). Indeed, human populations may be confronted to such an exposure in case of an accidental pollution of the environment. The general plasma parameters were unchanged after contamination, as was the gene expression of enzymes of cholesterol biosynthesis and storage. In the catabolism pathway, CYP7A1, CYP27A1, and CYP8B1 mRNA levels were unaffected by depleted uranium. However, the gene expression of CYP7B1 was decreased. Conversely, the mRNA levels of transporters ABC A1 ABC G5/G8 were increased. Thus, depleted uranium affects the expression of genes involved in cholesterol hepatic metabolism but induces no pathological phenotype in rats after chronic ingestion.
Contamination; Uranium; Liver; Cholesterol; Cytochrome P450
Cytochromes P450 are key enzymes for the conversion of cholesterol into bile acids that occurs in the liver [1]. More specifically, CYP7A1 initiates the neutral pathway of the biosynthesis of bile acids, while CYP27A1 initiates the acidic pathway. CYP7B1 is also involved in the acidic pathway, whereas CYP8B1 is common to both pathways and controls the ratio hydrophylic: hydrophobic bile acids. Another CYP is involved with cholesterol: CYP51, which belongs to the biosynthesis pathway.
Cholesterol metabolism has been shown to be modulated by depleted uranium (DU), a mildly radioactive heavy metal that could be released in the environment following industrial accidents or military use. Following an intraperitoneal injection of DU in rats [2], an increase of the hepatic CYP27A1 enzymatic activity and of FXR and LXRa mRNA levels was seen, as well as a decrease of 7a-hydroxycholesterol (oxysterol) plasma level. After a chronic ingestion of DU, cholesterol cerebral metabolism displayed also modifications at gene expression level [3]. Indeed, an increase of CYP46A1 (catabolizing cholesterol into 24S-hydroxycholesterol (oxysterol) in the brain) was found, as well as an increase in HMGCoA Synthase, which plays an important role in the biosynthesis of cholesterol. Moreover, the mRNA levels of several actors of the cholesterol transport system were also increased (SR-B1, ABC A1, ApoE) after chronic ingestion. These previous results highlight the fact that DU can modulate cholesterol metabolism in the brain, which is independent from the rest of the body in this respect. In this regard, the question arises about the effects of the same contamination on cholesterol metabolism in the liver. Indeed, the liver is the key organ for cholesterol homeostasis at body level because of its central role in lipoprotein metabolism and of its unique ability to eliminate the excess cholesterol through the biosynthesis of bile acids. A severe disruption at this level may lead to various pathologies such as cholestasis, cholelithiasis or atherosclerosis. In addition to this, depleted uranium has been shown to accumulate in the liver [4], where it can exert both its chemical and radiological toxicity
This paper presents the effects on cholesterol metabolism in rats ingesting a low dose of DU for 9 months (approximately equivalent to 20 years of human lifetime). Gene expression of cholesterol-catabolizing CYP7A1, CYP27A1, CYP7B1, CYP8B1 [5], as well as associated nuclear receptors [6] (LXRα, FXR, PPARα, and SREBP 2 was analyzed. Expression levels of ACAT 2 (cholesterol esterification), as well as HMGCoA Reductase and CYP51 (cholesterol biosynthesis) were also measured. The gene expression study was completed with SRB1 and LDLr (receptors to lipoproteins), Apo lipoprotein B and ABC A1, ABC G5, and ABC G8 (cholesterol transport) [7] (Figure 1).

Figure 1: Diagram of the main cholesterol metabolic pathways in the liver.
Male Sprague-Dawley rats (12 weeks, ~300 g) obtained from Charles River Laboratories (L’arbresle, France) were divided into two groups (n=10). For 9 months, rats of the experimental group daily received mineral water supplemented with depleted uranyl nitrate (UO2 (NO3 )2 , Cogema, Pierrelatte, France), at 40 mg/L. This concentration is not nephrotoxic and provides an internal contamination at low level of radiations. The control group was given uncontaminated mineral water.
All experimental procedures were approved by the Animal Care Committee of the Institute of radioprotection and Nuclear Safety, and complied with French regulation for animal experimentation (Ministry of Agriculture Act No. 87-848, October 19th 1987, modified May 20th 2001).
Gene expression levels were determined by real-time RT-PCR using SYBR Green PCR master mix (Applied Biosystems, Courtaboeuf, France) and specific primer sequences on an ABI Prism 7900 Sequence Detector (Applied Biosystems, Courtaboeuf, France). Hypoxanthine-guanine phosphoribosyltransferase (HPRT) was used as housekeeping gene.
Results are reported as mean ± SEM and are considered significant when p ≤ 0.05. Statistical analysis was performed using the unpaired Student’s t-test.
The general health status of the DU-contaminated rats was preserved at the end of the procedure. No difference was recorded in the body weight gain between both groups. The lipidic profile in the plasma was unchanged after the contamination, as were the levels of plasmatic markers of hepatic and renal integrity and function (data not shown).
It has been shown that a chronic ingestion of DU induces the gene expression of CYPs involved in xenobiotics metabolism in the liver. In this regard, the mRNA levels of others CYPs, involved in cholesterol hepatic metabolism were first measured in this study. There was no difference between both groups concerning the gene expression of CYP51 (involved in cholesterol synthesis), nor in that of CYP7A1 and CYP27A1, which initiate the classical and the alternate pathways of the biosynthesis of bile acids, respectively. However, a diminution of the mRNA level of CYP7B1 (involved in the alternate pathway) was recorded in the contaminated group (-31%, p=0.008, see Figure. 2). No significant modification was seen in the gene expression of ACAT 2, the enzyme that esterifies cholesterol for storage.
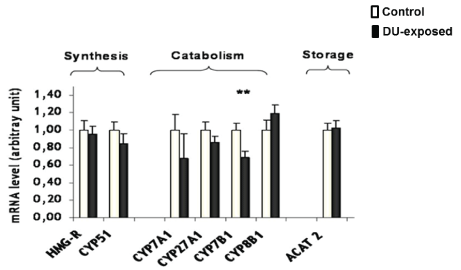
Figure 2: Gene expression of enzymes involved in hepatic cholesterol metabolism measured by real-time PCR in control or DU-exposed rats. The results are expresed as a ratio to HPRT mRNA level. Data are expressed as mean ± SEM, **p<0.01 significantly different from control.
Beside the synthesis, catabolism, and storage pathways, the cholesterol transport system was also studied (Figure 3). Whereas the lipoprotein receptors and the apolipoproteins mRNA levels were similar between the control and DU-exposed rats, some modifications were seen in the ABC transporters. Indeed, the gene expression of ABC A1 (effluxing cholesterol towards the general circulation) was increased by 54% in the contaminated rats (p<0.001). In the same time, the mRNA levels of both ABC G5 and ABC G8 were increased by 109% and 144% respectively (p<0.001). This is most interesting since these ABC transporters function as a heterodimer to transfer the cholesterol out the hepatocytes directly into the bile.

Figure 3: mRNA expression levels of transporters and membrane receptors involved in hepatic cholesterol metabolism measured by realtime PCR in control or DU-exposed rats. The results are expressed as a ratio to HPRT mRNA level. Data are expressed as mean ± SEM, ***p<0.001 significantly different from control.
Finally, the transcription factors regulating cholesterol metabolisms were also studied (Figure 4). A slight increase in RXR mRNA level was recorded (+23%, p=0.012). However, since the gene expression of no RXR heterodimer partner was modified, it is supposed that this modulation is either physiological, or linked to another metabolism in which RXR is involved.
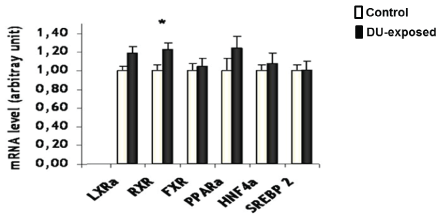
Figure 4: mRNA expression levels of nuclear receptors involved in hepatic cholesterol metabolism measured by real-time PCR in control or DU- exposed rats. The results are expressed as a ratio to HPRT mRNA level. Data are expressed as mean ± SEM, *p<0.05 significantly different from control.
Although these effects are restricted to the gene expression level, it might suggest a possible decrease in the rate of the production of bile acids by the alternate pathway due to the decrease in the CYP7B1 mRNA level. However, the increase of the gene expression of both ABC A1 and the heterodimer ABC G5/G8 may compensate this effect by an increase of cholesterol efflux out of the hepatocytes, both towards the general circulation and the bile. However, the increase of the ABC transporters is of greater magnitude than the diminution of CYP7B1. Therefore, it can also be considered that the prime modification affects the transporters and that the compensation is the other way around. However, other experiments are required to validate one hypothesis over the other.
This study shows for the first time that cholesterol metabolism is affected at gene expression level in the liver of rats submitted to a chronic ingestion of low levels of depleted uranium. However, these changes do not upset the general cholesterol homeostasis in the contaminated animals and do not seem to induce deleterious effects in adult rats. To confirm the harmlessness of depleted uranium in our experimental conditions, the use of other model more susceptible to external perturbations (such as growing pups and hyperlipidemic mice) should be helpful.
This study was financially supported by the Institute for Radioprotection and Nuclear Safety (ENVIRHOM research program) and by the Direction Générale des Armées (CER # 2006.94.0920).
Download Provisional PDF Here
Article Type: Research Article
Citation: Souidi M, Racine R (2017) Exposition of Uranium Modulates of the Expression of Cyps and Transporters Involved in Cholesterol Metabolism in Rat Liver. J Environ Toxicol Stu 1(1); doi http://dx.doi.org/10.16966/2576-6430.103
Copyright: © 2017 Souidi M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access