Introduction
Metformin is used to control blood glucose in patients with type 2
diabetes mellitus worldwide. A statement of the American Diabetes
Association (ADA) and the Europe Association for the Study of Diabetes
(EASD) recommends metformin as the first line of hypoglycemic agent
[1]. Metformin improves insulin sensitivity and is thus important in the
management of traditional cardiovascular risk factors such as a high
hemoglobin (Hb) A1c level, dyslipidemia, hypertension and central
obesity, all of which are associated with insulin resistance [2]. The
United Kingdom Prospective Diabetes Study demonstrated that the
risk of cardiovascular morbidity and mortality was reduced in patients
with type 2 diabetes mellitus receiving intensive glucose control using
metformin [3]. Thus, metformin improves not only blood glucose but also
cardiovascular morbidity and mortality.
Prevention of macrovascular complications is very important
for the treatment of diabetes. There are some surrogate markers of
atherosclerosis in clinical situation. Arterial stiffness is a useful surrogate
marker of atherosclerosis. Brachial-ankle PWV (baPWV) has been used
to evaluate arterial stiffness or atherosclerosis in diabetic patients. An
arterial stiffness parameter called cardio-ankle vascular index (CAVI)
was developed as a marker of arteriosclerosis involving the aorta, femoral
artery and tibial artery [4]. CAVI is measured from an electrocardiogram,
phonocardiogram, brachial artery waveform and ankle artery waveform
and is adjusted for blood pressure based on the stiffness parameter β [5].
CAVI is independent of blood pressure and has adequate reproducibility
for clinical use, whereas baPWV is dependent on blood pressure [4].
Although arterial stiffness can be evaluated by measuring either baPWV
or CAVI, CAVI is superior to baPWV as an index of arterial stiffness in
patients who have undergone coronary angiography [5]. A report has
shown that CAVI is useful for the detection of atherosclerotic diseases
[4]. Some hypoglycemic agents improve CAVI within 6 months in several
clinical studies [6-8]. Thus, CAVI is a very useful marker for evaluating
atherosclerosis in diabetic patients.
In Japan, the maximum dose of metformin had been 750 mg/day for a
long time. However, physicians have been able to prescribe metformin at
a maximum dose of 2250 mg/day since 2010. Metformin 250 mg/tablet
had been the only available formulation in Japan until metformin 500 mg/
tablet was released at 2013. Many diabetic patients take metformin 250
mg twice daily, but now physicians can switch from metformin 250 mg
twice daily to metformin 500 mg once daily by taking only one tablet.
With the release of dipeptidyl peptidase-4 (DPP-4) inhibitors and sodium
glucose cotransporter (SGLT)-2 inhibitors in recent years, many types of
hypoglycemic agents are now available, and some patients are treated with
multiple agents. If metformin 500 mg once daily has the same efficacy as
metformin 250 mg twice daily, physicians can reduce the number of tablets
which may increase compliance. However, because of the short half-life
of metformin, metformin 500 mg once daily has a risk of deteriorating
blood glucose control and macrovascular complications compared with
metformin 250 mg twice daily [9].
In the present study, we investigated the effects of metformin 500 mg
once daily on blood glucose control and atherosclerosis, and compared
with metformin 250 mg twice daily.
Subjects and Methods
Subjects
The study was conducted in accordance with Helsinki Declaration and
was approved by the institutional review board of Sakura Hospital Toho
University Medical Center (No. 2013-039). Before participation, the purpose
of the study was explained to each subject, and consent was obtained for both
participation in the study and for release of the study data.
A randomized open-label study was performed. We enrolled 70
patients with type 2 diabetes mellitus, who had been treated with
metformin 250 mg twice daily for at least 3 months Sakura Hospital and
whose HbA1c had been steady for 3 months. The exclusion criterion was
patients with renal dysfunction [estimated glomerular filtration (eGFR)
<30 ml/min/1.73 m2
]. We divided the patients into 2 groups by simple
randomization using a closed envelope. One group was switched from
metformin 250 mg twice daily to metformin 500 mg once daily (once
daily group, n=35), and the other group continued to take metformin
250 mg twice daily (twice daily group, n=35). Table 1 shows the clinical
characteristics of the subjects at baseline. The subjects were observed for
6 months, and the following parameters were measured before and after 6
months: body weight (BW), body mass index (BMI), fasting blood glucose
(FBG), glycosylated, hemoglobin (HbA1c), aspartate transaminase (AST),
alanine transaminase (ALT), γ-glutamyl transpeptidase (γ-GTP), blood
urea nitrogen (BUN), serum creatinine, eGFR, serum total cholesterol
level (TC), serum triglycerides level (TG), serum high-density lipoproteincholesterol
level (HDL-C) and serum low-density lipoprotein-cholesterol
level (LDL-C). Systolic blood pressure (SBP), diastolic blood pressure
(DBP) and CAVI were also measured before and after 6 months. During
this study, all patients maintained the same diet and exercise therapies and
did not change medications. All subjects received nutritional guidance
from a dietitian every month. The dietitian analyzed the meals of the
patients and suggested changes if necessary.
Body weight measurement and blood sampling
Body weight was measured and blood samples were collected in the
morning after 12 hours of fasting. Serum was separated within 1 hour, and
the sample was used for measurements of HbA1c, AST, ALT, γ-GTP, BUN,
serum creatinine, eGFR and serum lipids.
Measurement of HbA1c and plasma lipid concentrations
For HbA1c measurement, blood was collected in tubes containing
ethylene diamine tetra acetic acid (EDTA). The stable and unstable fractions
of HbA1c were measured by a high-pressure liquid chromatography
method using Hi-Auto A1c (Kyoto Daiichi Kagaku, Kyoto, Japan). Data of
the stable form were used in the present analysis. The data of HbA1c was
expressed as the value of the National Glycohemoglobin Standardization
Program (NGSP).
Plasma TC and TG levels were measured enzymatically using kits from
Nippon Shoji Co., Ltd. (Osaka, Japan) and a HITACHI 7150 analyzer
(Hitachi, Ltd., Tokyo, Japan). HDL-C was measured by a selective
inhibition assay (Daiichi Pure Chemicals Co., Ltd., Tokyo) [10]. Serum
LDL-C levels were calculated using the Friedewald formula.
Measurement of CAVI, systolic and diastolic blood pressure
CAVI is obtained by measuring blood pressures and pulse wave velocity
(PWV) according to the following formula: CAVI = a{(2ρ/∆P) × ln(Ps/
Pd)PWV2
}+b, where Ps is systolic blood pressure; Pd is diastolic blood
pressure; PWV is pulse wave velocity; ∆P is Ps - Pd; ρis blood density, and
a and b are constants. The details of CAVI and the measurement of CAVI
are described in our previous reports [4,7].
In the present study, CAVI was measured using a VaSera CAVI
instrument (Fukuda Denshi Co., Ltd., Tokyo, Japan) as described
previously [4]. Systolic and diastolic blood pressures were measured at the
time of CAVI measurement.
Statistical analysis
Data were expressed as mean ± SD. Normal distribution was tested
by the Shapiro-Wilk test. Some data were not normally distributed, and
normality was obtained by logarithmic transformation. Statistical analysis
was performed using the Student’s t-test and ANOVA. All analyses were
performed using the JMP version 9.0 (SAS, Cary, NC, USA). P values
<0.05 were considered significant.
Results
Baseline characteristics in once daily group and twice daily group
There were no significant differences in baseline parameters between
once daily group and twice daily group (Table 1). Use of other hypoglycemic
agent was also almost the same in the two groups (Table 1), except that use
of thiazolidinediones was four times higher in twice daily group than in
once daily group. Only one patient took glinide in this study (Table 1). No
patients used glucagon-like peptide-1 (GLP-1) agonists. No patients took
SGLT-2 inhibitors, because all subjects in this study were registered before
SGLT-2 inhibitors were available in Japan.
Comparisons of changes of clinical parameters after 6 months in two groups
Comparison between the two groups revealed that the change in FBG
was -1.51 ± 62.55 mg/dl in once daily group and +14.91 ± 57.64 mg/dl
in twice daily group, but the difference was not significant (P=0.2572)
(Table 2). Serum creatinine and eGFR apparently deteriorated slightly in
once daily group and improved slightly in twice daily group. However,
changes of these two parameters were not significantly different between
two groups (Table 2). Changes in other clinical parameters also were not
significantly different between two groups (Table 2).
Metformin-related adverse effects such as lactic acidosis, anxiety and
confusion signs were not observed in any of the patients.
Change in HbA1c in once daily group and twice daily group
In the once daily group, HbA1c was 7.58 ± 0.93 at baseline and 7.83 ±
1.13 at 6 months (Figure 1A). HbA1c in twice daily group was 7.68 ± 1.00
at baseline and 7.81 ± 1.02 at 6 months (Figure 1B). The changes in both
groups were not significant (P=0.0849 in once daily group, P=0.2523 in
twice daily group). The changes in HbA1c during this study are shown in
Figure 1C. The change in HbA1c was not significantly different between
once daily and twice daily groups (+0.25 ± 0.66 vs. +0.13 ± 0.68, P=0.5247).
Change in CAVI in once daily group and twice daily group
In once daily group, CAVI was unchanged after 6 months (from 9.50 ±
1.00 to 9.54 ± 1.11, P=0.8504) (Figure 2A). CAVI in twice daily group also
did not change significantly (P=0.2964) (Figure 2B). The changes in CAVI
were not significantly different between once daily and twice daily groups
(+0.04 ± 0.73 vs. +0.11 ± 0.62, P=0.5815) (Figure 2C).
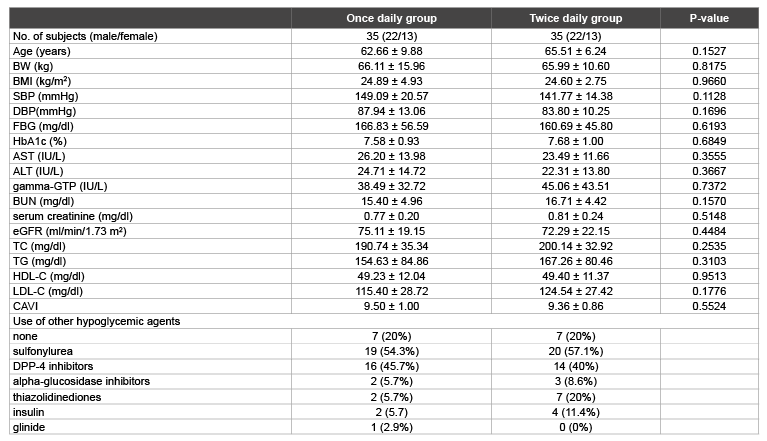
Table 1: Comparison of baseline characteristics in the two groups
Data are presented as mean ± SD or number (%). BW: Body Weight; BMI: Body Mass Index; SBP: Systolic Blood Pressure; DBP: Diastolic Blood
Pressure; FBG: Fasting Bood Glucose; HbA1c: Glycosylated hemoglobin; AST: Aspartate Transaminase; ALT: Alanine Transaminase; GTP: Glutamyl
Transpeptidase; BUN: Blood Urea Nitrogen; eGFR: estimated Glomerular Filtration Rate; TC: Total Cholesterol; TG: Triglycerides; HDL-C: High Density
Lipoprotein-Cholesterol; LDL-C: Low Density Lipoprotein-Cholesterol; CAVI: Cardio-Ankle Vascular Index; DPP-4: Dipeptidyl Peptidase-4.
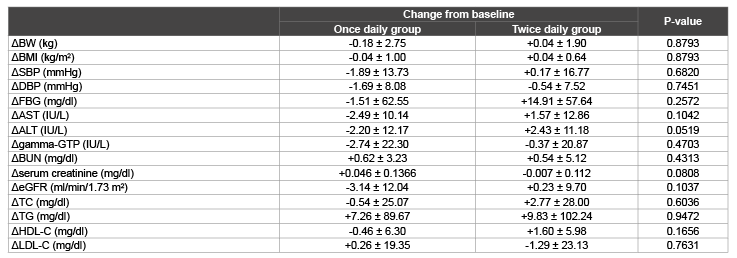
Table 2: Comparison of the changes in clinical parameters after 6 months in the two groups
Data are presented as mean ± SD. Δ denotes the difference between the value at baseline and that after 6 months. Abbreviations are as in Table 1.
Comparison between male and female in each group
We investigated the sexuality effect or relationship between male and
female in this study. We compared the differences between male and
female in each group. At baseline, BW and HbA1c were significantly
different between male and female in once daily group (Table 3). However,
the change in BW, BMI, FBG, HbA1c and CAVI were not significantly
different between male and female in once daily group (Table 3). In twice
daily group, BW and CAVI were significantly different between male and
female at baseline. BW and BMI were significantly decreased in male
subjects after 6 months, but the change in FBG, HbA1c and CAVI were not
significantly different between male and female in twice daily group (Table 3).
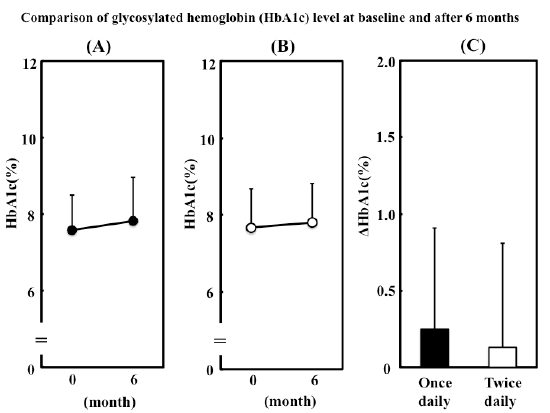
Figure 1: Comparison of glycosylated hemoglobin (HbA1c) level at
baseline and after 6months.
(A) Change in HbA1c in patients who switched to metformin 500 mg
once daily. (B) Change in HbA1c in patients who continued to take
metformin 250 mg twice daily. (C) Changes in HbA1c after 6 months in
two groups. Data are presented as mean ± S.D. Δ denotes the difference
between the value at baseline and that after 6 months.
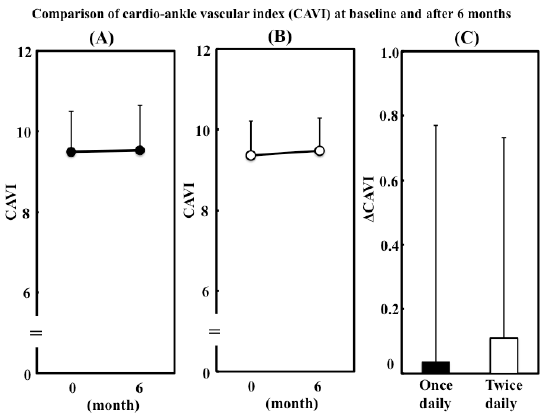
Figure 2: Comparison of cardio-ankle vascular index (CAVI) at baseline
and after 6 months.
(A) Change in CAVI in patients who switched to metformin 500 mg once
daily. (B) Change in CAVI in patients who continued to take metformin
250 mg twice daily. (C) Changes in CAVI after 6 months in two groups.
Data are presented as mean ± S.D. Δ denotes the difference between
the value at baseline and that after 6 months.
Discussion
In the present study, switching from metformin 250 mg twice daily to
metformin 500 mg once daily did not affect blood glucose control, and
also did not change CAVI that reflects arterial stiffness and is a surrogate
marker of atherosclerosis. Furthermore, there were no metforminrelated
adverse effects, and lipid metabolism and parameters of liver and
kidney function were also unchanged. There was no sexuality effect or
relationship between male and female in this study.
Metformin, an insulin sensitizer, has been shown to reduce the
incidence of myocardial infarction compared with sulfonylurea agents
in a large-scale study [3]. This result may be one of the reasons why
metformin is recommended as the first-line hypoglycemic agent. Patients
usually take metformin two or three times daily because of its short halflife.
In the present study, we compared the efficacy of metformin 500 mg
once daily with metformin 250 mg twice daily. Fasting blood glucose was
unchanged and HbA1c did not change significantly in once daily group.
The change in HbA1c was not significantly different between once daily
and twice daily groups. Hwang et al. [11] reported that glimepiride 2 mg
and metformin 500 mg once daily was as effective as glimepiride 1 mg
and metformin 250 mg twice daily. Although the half-life of glimepiride is
also relatively short, glimepiride and metformin once daily showed high
efficacy [11,12]. Our study using only metformin showed the same result
as Hwang et al. [11] who used glimepiride and metformin. In the Japanese
package insert for metformin, the Tmax, Cmax, AUC0-48 and T1/2 for 250 mg
are 1.9 h, 898 ng/ml, 4861 ng•hr/ml and 2.9 h; while the corresponding
data for 500 mg are 2.3 h, 1341 ng/ml, 8019 ng•hr/ml and 4.0 h. Other
reports on metformin 500 mg show Tmax of 2.4 h, Cmax of 1420 ng/ml, and
T1/2 of 3.16 h [13,14], and these figures are similar to those shown in the
Japanese package insert. Although the half-life is not remarkably different
between metformin 250 mg and 500 mg, Cmax and AUC0-48 of metformin
500 mg are clearly larger than those of metformin 250 mg. The large Cmax
and AUC0-48 may be a reason why metformin 500 mg once daily has the
same effectiveness in controlling blood glucose as metformin 250 mg
twice daily.
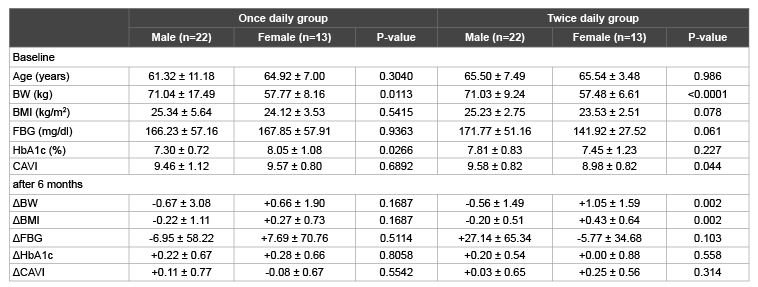
Table 3: Comparison between male and female in each group
Data are presented as mean ± SD. Δ denotes the difference between the value at baseline and that after 6 months. Abbreviations are as in Table 1.
Cardiovascular disease is the leading cause of death among type 2
diabetic patients [15]. Preventing macrovascular complications is a very
important goal for the treatment of diabetes. There are some surrogate
markers for atherosclerosis. Arterial stiffness is closely related to
atherosclerosis, and several reports show that CAVI is a useful surrogate
marker for atherosclerosis in patients with or without diabetes [16-18].
CAVI decreases through improvement of postprandial hyperglycemia or
insulin resistance in type 2 diabetes patients [6-8]. In the present study,
metformin 500 mg once daily did not change CAVI, and the changes in
CAVI in once daily and twice daily groups were not significantly different.
These results indicate that switching from metformin 250 mg twice daily
to metformin 500 mg once daily does not deteriorate atherosclerosis in
type 2 diabetes patients.
Some clinical studies have shown that metformin improves the
lipoprotein profile in patients with diabetes [19-21]. Metformin also
improves the qualities of LDL-cholesterol. Metformin enlarges LDL
particle size and reduces circulating malondialdehyde-modified LDL,
which is one of the oxidized LDLs [22,23]. Thus, metformin also affects
lipid metabolism. In the present study, metformin 500 mg once daily had
no negative effect on lipid metabolism compared with 250 mg twice daily.
The half-life of metformin is short, and metformin 500 mg once daily does
not deteriorate blood glucose concentration, arterial stiffness, or lipid
metabolism compared with metformin 250 mg twice daily.
Lactic acidosis is the most well known and serious adverse effect of
metformin. Lactic acidosis usually occurs due to drug overdose or in
some contraindicated conditions such as liver or kidney dysfunction [24].
For this reason, we excluded patients with kidney dysfunction (eGFR<30
ml/min/1.73 m2
). Metformin at 500 mg/day is not an overdose, but
administration of 500 mg per dose may be high for some patients. In the
present study, none of the patients had lactic acidosis. Therefore, metformin
500 mg once daily is safe in patients without kidney dysfunction.
AST and ALT showed apparent decreases in once daily group and
increases in twice daily group, although all the levels were within normal
ranges. The changes in AST and ALT tended to be different between once
daily and twice daily groups (p=0.1042 and 0.0519, respectively), and the
difference was almost significant for AST. Metformin reduces hepatic
steatosis via activating AMP-activated protein kinase (AMPK) and
inactivating acetyl-CoA carboxylase (ACC) [25], which may improve liver
function. Since Cmax and AUC0-48 of metformin 500 mg are much higher
than those of metformin 250 mg, metformin 500 mg once daily may have
more potent effect on liver function compared with metformin 250 mg
twice daily.
There are two limitations to the present study. The study duration was
only 6 months. Thus, the long-term efficacy and safety of metformin 500
mg once daily is still unclear. Further investigation with longer evaluations
may be necessary. However, we were able to show that metformin 500 mg
once daily does not deteriorate blood glucose control, lipid metabolism, or
arterial stiffness for at least 6 months. Another limitation is that we did not
evaluate microvascular complications. Microvascular complications are as
important as macrovascular complications in diabetic patients. Therefore,
investigation of the progression of microvascular complications may be
necessary. However, HbA1c was almost unchanged in this study. Since
diabetic microvascular complications are strongly associated with HbA1c
[26], we speculate that microvascular complications might not be different
between two groups.
In summary, switching from metformin 250 mg twice daily to
metformin 500 mg once daily did not deteriorate blood glucose control or
arterial stiffness. Furthermore, no adverse effects including lactic acidosis
were observed associated with this switch. These results suggest that
metformin 500 mg once daily is safe and has the same efficacy compared
with metformin 250mg twice daily.
Disclosure
Potential conflicts of interest with any of the authors: None
This research received no specific grant from any funding agency in the
public, commercial or not-for-profit sectors.






