Abstract
An increased prevalence of cardiovascular problems is recorded due to aging. The importance of aging as a potential risk factor for cardiovascular diseases has been increasing in most of the developing countries since populations continue to become older. Age-associated unusual structural changes that develop in blood vessels have been reported such as stiffening of the vasculature and fibrosis. CaMKII is a protein kinase that is present all-over in the body. In this study, it is suggested that acute inhibition of CaMKII-mediated signaling may be an effective method to prevent agingassociated detrimental changes in isolated blood vessels in SD male rats. The inhibitory effect of CaMKII in aging-induced renal/carotid vascular dysfunction was studied using the selective inhibitor KN-93. Isolated ring segments of the renal or carotid arteries were suspended in organ-bath chambers for measurement of isometric tension. Responses to Phenylephrine (PE), Endothelin-1 (ET-1), carbachol and Sodium Nitroprusside (SNP) were determined by measurement of changes in isometric tension. Results from this study showed abnormal vascular responses to vasoactive agonists in the vessels obtained from aged rats. Vasoconstrictor responses to phenylephrine were potentiated, while vasodilator responses to carbachol were reduced in blood vessels obtained from aged rats, in comparison to responses in young rats. Acute incubation of the blood vessels with KN-93 (10-5 M), an inhibitor of CaMKII, resulted in significant improvement in vascular function to both vasoactive agonists. Our results indicated that activation of CaMKII pathway contributes to aging-induced vascular dysfunction. Therefore, development of novel therapies that inhibit CaMKII pathway may prevent or reduce development of vascular abnormalities induced by aging.
Keywords
Carbachol; KN-93; Vascular reactivity; Vasoconstriction
Introduction
The importance of aging as a potential risk factor for all cardiovascular diseases has been increasing as in most of the developing countries the populations persist to grow older. It is expected that by the year 2030, 70 million people above the age of 65 years will be in the United States alone. An increase in the incidence of cardiovascular diseases has been reported as a result of aging [1]. It has been documented that there are age-associated remarkable structural modifications that develop in the blood vessels and the heart. For example, stiffening of the vasculature, fibrosis and potentiation in the thickness of the left ventricular wall are different structural changes that have been expressed with aging [1]. There are also many functional abnormalities in the cardiovascular system induced by aging such as changes in the contractility, sympathetic signaling and heart rate. In the heart, the mechanisms responsible for prevention of damage and to correct the injury are found to turn to be increasingly inadequate with aging. These defective mechanisms result in prominent augmented impaired function [1,2]. Due to aging, the defective function in the vasculature and the heart would lead to a progressive enhancement in the occurrence of cardiovascular diseases [1].
CaMKII is a Ser/Thr-directed protein kinase that is present everywhere all-over (ubiquitous). It is observed that CaMKII gets switched on by binding to the Ca2+-calmodulin complex due to an increase in the free calcium ions (Ca2+) located intracellular [3]. As a result, the process of “autophosphorylation or oxidation of CaMKII” may continue its action despite of the amount of intracellular calcium ions, which may cause phosphorylation of several positions [4,5]. Several studies have previously reported that inhibition of signal transduction involving CaMKII can prevent development of diabetes-induced vascular dysfunction [6-8]. Our published data suggested that CaMKII is an essential mediator in the development of diabetic vascular abnormality [6,8]. In this study, it is suggested that acute inhibition of CaMKII-mediated signaling may be an effective approach to prevent injury-promoting factors in isolated blood vessels associated with aging in SD male rats. In this study, the inhibitory effect CaMKII using the selective inhibitor KN-93 was investigated on aging-induced renal/carotid vascular dysfunction. Vascular function was examined by studying the vascular reactivity of isolated renal and carotid arteries to vasoactive agonists associated with acute inhibition of CaMKII by the selective inhibitor KN-93.
Materials and Methods
Experimental procedures
Male SD rats were used in this study. Animals are divided into two groups. Group 1 are control animals (10 weeks old). Group 2 are older animals (30 weeks old) (n=30 animals per group). The investigation conforms to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, Revised 1985) and is approved by Kuwait University Research Administration as the use of animals is in accordance with Institute for Laboratory Animal Research Guide for Care and Use of Laboratory Animals.
Isolation of the renal and carotid arteries
Rats were sacrificed by decapitation under light ether anesthesia. The renal and carotid arteries from control or aged rats were isolated carefully and transferred to a Petri dish containing oxygenated KrebsHenseleit solution (KH solution). The vessels were cut into 5 mm segments. The vascular rings were mounted in organ-baths using tungsten wires.
Force measurements
Isolated ring segments of the renal or carotid arteries were suspended in organ-bath chambers containing 25 ml Krebs Henseleit (KH) solution at pH 7.4, for measurement of isometric tension. The composition of KH-solution is as follows (mM): NaCl (118.3), KCl (4.7), CaCl2 (2.5), MgSO4(1.2), NaHCO3(25), KH2PO4 (1.2) and glucose (11.2). The tissue bath solution was kept at 37°C and aerated with 95% oxygen and 5% carbon dioxide mixture. Reactivity of the isolated blood vessels was investigated by measurement of changes in the isometric tension to vasoactive agonists using computerized Automatic organ bath LSI Letica Scientific Instruments (Powerlab/8sp ADI insturments, Panlab, Spain). The preparations were mounted and left for 45 min with changing KH solution at 15 min intervals. Thereafter, a pretension of 0.5 g was applied to the renal artery ring segments, while carotid artery segments were preloaded with a pretension of 1.0 gm. All the preparations were allowed to stabilize for 45 min until a stable baseline tone is obtained.
Effects of vasoconstrictor agonists
Following the equilibration period, cumulative concentration response curves to Phenylephrine (PE) or endothein-1 (ET-1) (10-9-10-5 M), were constructed. The agonists were sequentially added to the organ baths and the maximal increase in tension will be recorded [3]. The different concentrations of the agonists were added successively to the organ baths to establish the vasoconstrictor responses. Each concentration of the drug was applied to the organ bath and left until the response was stable, before adding the following concentration.
Effects of vasodilator agonists
The vasodilator responses to carbachol and Sodium Nitroprusside (SNP) were determined using freshly mounted isolated blood vessels. The vessels were first tested with a test dose of noradrenaline (10-6 M) to check the viability of the tissues. Thereafter, the tissues were washed with KH solution and organ baths were then refilled with fresh KH solution in which the blood vessels were left for 30 minutes to stabilize. The renal or carotid artery segments in these experiments were contracted with a submaximal concentration of NE (10-6 M). After achieving a steady level of tension, cumulative concentration response curves to carbachol (10-9-10-4 M), and SNP (10-9-10-4 M) were established. Each concentration of the drug was given and left to stabilize before adding the next concentration. The vasodilator response was measured as per cent change in the maximal initial contraction induced by NE (10-6 M).
Effect of acute incubation with KN-93 on the vascular reactivity of renal and carotid arteries from control and aged male SD rats
Another set of experiments is performed in order to investigate the effect of acute incubation with the selective CaMKII inhibitor, KN-93, on the vascular responses to the vasoactive agonists in the renal and carotid arteries isolated from both adult and aged SD rats.
Effects of vasoconstrictor agonists after acute incubation with KN-93
As described earlier, cumulative concentration response curves to Phenylephrine (PE) or ET-1 (10-9-10-5 M), were constructed. KN-3 (10-5 M) was added to the organ baths and left in contact with the tissues for 30 minutes. After the incubation period, cumulative concentration response curves to Phenylephrine (PE) or ET-1 (10-9-10-5 M), were established in the presence of the CaMKII inhibitor, KN-93. The agonists were added to the organ baths and the maximal increase in tension was recorded as explained in the previous section. Experiments were performed using different tissues to examine the responses to the agonists in the presence of the inhibitor.
Effects of vasodilator agonists after acute incubation with KN-93
The vasodilator responses to carbachol and Sodium Nitroprusside (SNP) were determined in freshly isolated carotid and renal artery as described earlier. The vessels were then washed and incubated in KN93 (10-5 M), which were applied to the organ baths and kept for 30 minutes. While in the presence of KN-93, the renal or carotid artery segments were pre-contracted with a submaximal concentration of NE (10-6 M) to construct cumulative dose response curves to carbachol (10-9-10-4 M), and SNP (10-9-10-4 M) as has been explained earlier. Different tissues were used in order to test the responses of the agonists in the presence of KN-93.
Drugs
(±)-Noradrenaline-bitartrate, KN-93, endothelin-1, sodium nitroprusside, and carbachol were obtained from Sigma Biochemicals (St. Louis, MO, USA). All drugs were dissolved in water or ethanol if needed.
Statistical Analysis
Results are analyzed using Graph Pad Prism software. Data are presented as mean ± SEM of n number of experiments. Analysis of variance is performed by comparing Mean values and is followed by post hoc test (Bonferroni). The difference between the mean ± SEM is considered to be significant when the P-value is less than 0.05.
Results
Effects of vasoconstrictor agonists after acute incubation with KN-93
Cumulative concentration response curves were established for PE in tissues isolated from young or aged rats. As shown in figure 1, PE-induced contractile responses in the carotid artery isolated from old rats were significantly potentiated compared to responses in vessels from young rats. Incubation with KN-93 (10-5 M) resulted in significant reduction in PE-induced contractions in the isolated carotid artery ring segments from old rats (p<0.05). Similarly, PE-induced contractile responses were increased in the renal artery isolated from old rats. Incubation of renal artery ring segments from old rats with KN-93 (10-5 M) caused significant reduction in PE-induced contractile responses (Figure 2).
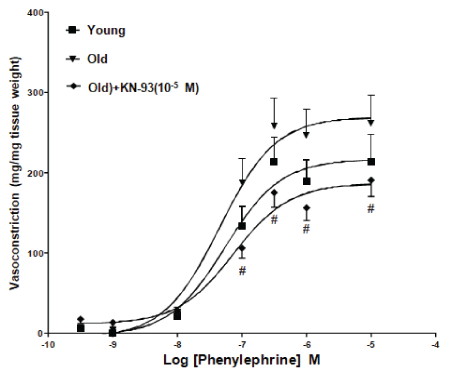
Figure 1: KN-93 attenuated Phenylephrine (PE)-induced contractile responses in the carotid artery isolated from old rats. Effect of incubation with KN-93 (10-5 M) on PE-induced contractions in the isolated carotid artery ring segments from old rats. Mean ± SEM (n=5- 6). #Values significantly different compared to old rats (p<0.05).
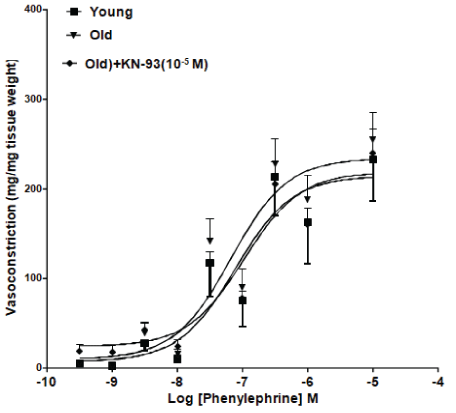
Figure 2: KN-93 attenuated Phenylephrine (PE)-induced contractile responses in the renal artery isolated from old rats. Effect of incubation with KN-93 (10-5 M) on renal carotid artery ring segments from old rats.
Effects of vasodilator agonists after acute incubation with KN-93
Cumulative concentration response curves were established for carbachol and SNP in tissues isolated from young or aged rats. Effects of incubation with KN-93 on vasodilator responses to carbachol and SNP were investigated. Carbachol-induced vasodilation was attenuated in both renal and carotid arteries from old animals. Acute incubation with KN-93 improved the impaired responses to carbachol in the carotid and renal artery of old rat (p<0.05) (Figures 3 and 4). However, vasodilator responses to SNP were not significantly different in the different study groups.

Figure 3: KN-93 improved the impaired responses to carbachol in the carotid artery of old rat. Effect of incubation with KN-93 (10-5 M) on relaxant responses induced by carbachol in the isolated carotid artery ring segments from old rats. Mean ± SEM (n=5-6). *Values significantly different compared to control young rats. #Values significantly different compared to old rats (p<0.05).
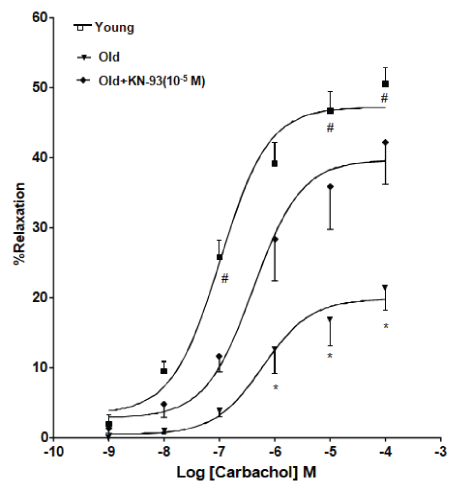
Figure 4: KN-93 improved the impaired responses to carbachol in the renal artery of old rat. Effect of incubation with KN-93 (10-5 M) on relaxant responses induced by carbachol in the isolated renal artery ring segments from old rats. Mean ± SEM (n=5-6). *Values significantly different compared to control young rats. #Values significantly different compared to old rats (p<0.05).
Discussion and Conclusion
Results from this study indicated that there was a significant abnormal vascular reactivity in both renal and carotid arteries from aged rats in response to vasodilator or vasoconstrictor agonists. The augmented vasoconstrictor response to PE was significantly reduced by acute incubation with KN-93. Similarly, the significant attenuation in carbachol-induced vasodilator responses in tissues form aged rats was corrected by acute incubation with KN-93. Thus our findings suggest that inhibition of CaMKII signaling pathway may prevent or retard the functional abnormalities in the vascular system that are induced by aging. Our findings are in agreement with previous reports that indicated a role for CaMKII pathways in mediating diabetes-induced vascular dysfunction. It has already been reported that inhibition of CaMKII pathway can retard the development of vascular abnormalities in tissues isolated for type 2 diabetes rats or in hypertension [6-8].
Some reports provided evidence that suggests the endothelial Nitric Oxide Synthase (eNOS) is a phosphorylation target of CaMKII [9,10]. However, this was not supported by another study as CaMKII regulates eNOS activity [11]. Murthy S, et al., (2017) [12] reported that CaMKII was identified as a regulator of eNOS phosphorylation and NO production in endothelium in vitro. Their findings are in agreement with other results in smooth muscle and cardiomyocytes which have shown that CaMKII activation in vivo under baseline physiological conditions was low. In addition, pathological activation of CaMKII was reported in several disease conditions, such as diabetes and hypertension [13,14]. Cheng LC, et al., (2020) [15] reported that endothelial dysfunction was protected by activating the Akt/CaMKII/ AMPK/eNOS/NO signaling pathway in Torenia concolor Lindley var. Formosama Yamazaki Ethanolic Extract (TCEE) (Figure 5).
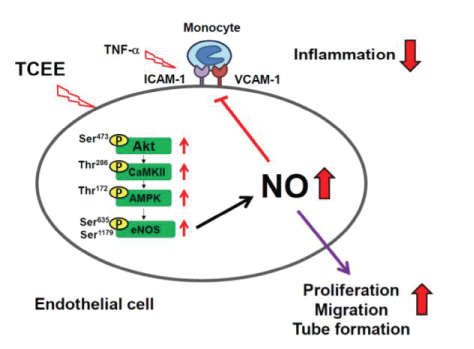
Figure 5: A schematic diagram showing the relation between CaMKII and eNOS activation. Attenuated endothelial dysfunction by activating the Akt/CaMKII/AMPK/eNOS/NO signaling pathway. Source: Cited from Cheng LC, et al., (2020) [15].
Therefore, it is highly probable that CaMKII contributes to endothelial-dependent vascular disease, which can be queried using our novel models of endothelial-specific CaMKII inhibition in vivo. This, together with the fact that CaMKII activity in these studies was examined using KN-93 calls for further clarification. Our results suggest that signal transduction involving CaMKII contributes to the development of abnormal vascular reactivity and renal dysfunction due to aging. We conclude that inhibition of CaMKII-mediated signaling could be an effective way to prevent the cardiovascular abnormalities that occur due to old age.
In summary, the present study indicates that blockade of CaMKII normalizes the altered vascular reactivity to carbachol and phenylephrine in the renal and carotid artery of aged rats, and suggests that selective inhibitors of this pathway may be beneficial in treatment of vascular complications associated with aging.
Acknowledgement
This study was funded by Kuwait University, Research Administration, Project No. MR02/16. The authors of this manuscript have no conflict of interest to declare.
Article Information
Article Type: RESEARCH ARTICLE
Citation: Yousif MHM, Makki B (2022) Attenuation of Aging-Induced Vascular Dysfunction by Inhibition of Ca2+/Calmodulin Protein Kinase II (CaMKII) in Isolated Renal and Carotid Rat Arteries. J Drug Res Dev 8(1): dx.doi.org/10.16966/2470-1009.169
Copyright: © 2022 Yousif MHM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 07 Feb, 2022
Accepted date: 17 Feb, 2022
Published date: 23 Feb, 2022






