Figure 1: Chemical structure of samples
(a) α-Cyclodextrin (αCD), (b) β-Cyclodextrin (βCD), and (c) γ-Cyclodextrin (γCD)


Akiho Mitsumori1 Sachie Narumi1 Yutaka Inoue1* Isamu Murata1 Shun-ichi Mitomo2 Yukiko Negishi2 Ikuo Kanamoto1
1Laboratory of Drug Safety Management, Faculty of Pharmacy and Pharmaceutical Sciences, Josai University, Japan*Corresponding author: Yutaka Inoue, Laboratory of Drug Safety Management, Faculty of Pharmacy and Pharmaceutical Science, Josai University, 1-1 Keyakidai, Sakado-shi, Saitama, 3500295, Japan, Tel: +81-49-271-7317; Fax: +81-49-271-7317; E-mail: yinoue@josai.ac.jp
The use of an electrochemical detector (ECD) and core-shell columns as a new quantitative method for the analysis of α-, β-, and γ-cyclodextrin(αCD, βCD, and γCD, respectively) was investigated, and simple quantitative methods and appropriate measurement conditions were developed. The retention times of αCD, βCD, and γCD were determined to be 6.5, 12.9, and 10.4 min, respectively, when measured using an ECD, a 50°C column temperature, 0.5 mL/ min flow rate, 20 µL injection volume, 0.3 mol/L NaOH/0.3 mol/L CH3 COONa=1/1 mobile phase, and an S-40/60=St (styrene)/DVB (divinylbenzene)-5TMA (trimethylamine) core-shell column. The calibration curves of αCD, βCD, and γCD demonstrated good linearity (R2 >0.998). The repeatability, precision, and accuracy were acceptable (relative standard deviation <5% and Recovery=100 ± 5%). The limit of detection and quantification of αCD were determined to be 0.26 and 0.81 µg/mL, those of βCD were 7.65 and 23.19 µg/mL, and those of γCD were 3.87 and 11.74 µg/mL, respectively. Thus, analysis of the cyclodextrins αCD, βCD, and γCD could be carried out by a simple method using an ECD and a core-shell column.
Cyclodextrins; ECD; Core-shell; Limit of quantification
Cyclodextrin (CD) is a cyclic oligosaccharide, which is α (1 → 4) glycosidically linked to D-glucopyranose. It is classified as α-Cyclodextrin (αCD), β-Cyclodextrin (βCD), or γ-Cyclodextrin (γCD) according to the number of glucose units [1]. In recent years, detectors and instruments such as evaporation light scattering detectors (ELSD), refractive index detectors (RI), and mass spectrometers (MS) have been used for CD quantification [2-5]. However, these methods are not suitable for low concentration measurements due to their large quantitative limit (about 50 mg/mL) [6].
Recently, an electrochemical detector (ECD) has been used for the quantitative analysis of saccharides such as monosaccharides and rare sugars [7]. An ECD operates by detecting the amount of electricity flowing during a redox reaction. In addition, when a basic solvent is used for the mobile phase, the alcoholic hydroxyl groups of saccharides are dissociated, and thus the ECD detects saccharides present as ionic molecules in anion exchange chromatography. The advantages of the ECD are: 1) it can be used for substances having alcoholic hydroxyl groups, 2) it has high sensitivity, and 3) it does not require additional sample preparation such as derivatization.
Core-shell columns are common separation columns used for the analysis of saccharides [8]. The core-shell column comprises a porous outer layer and a hard nucleus inner layer. The advantage of the coreshell column over a completely porous column is that mobile phases and samples are not adsorbed by the hard nucleus, and as a result the diffusion path is shorter, the elution time of the sample is shortened, and sharp peaks are obtained [9].
Improving the concentration detection of CD by a simple method using an ECD would be useful as a novel assay. The aim of this research was to investigate the use of an ECD and core-shell columns as a new quantitative method for the analysis of αCD, βCD, and γCD, and to develop simple quantitative methods and appropriate measurement conditions.
Cyclodextrins αCD, βCD, and γCD were donated by Cyclo Chem Co., Ltd. (Figure 1). Other reagents were of a special commercial grade from Wako Pure Chemical Industries.

Figure 1: Chemical structure of samples
(a) α-Cyclodextrin (αCD), (b) β-Cyclodextrin (βCD), and (c) γ-Cyclodextrin (γCD)
Stock solutions (500 µg /mL) of αCD, βCD, and γCD (50 mg) were prepared with distilled water. From this stock solution, further samples were prepared at five different concentrations from 5 to 500 µg/mL. In addition, to calculate the recovery and relative standard deviation, a solution of known concentration (50 µg/mL) of each CD was prepared.
αCD, βCD and γCD at each concentration of 500 µg/mL was used to find the suitable measurement conditions. Sample measurements were performed using an electrochemical detector (ECD: SU-300, DKK-TOA), 50°C column temperature, 0.5 mL/min flow rate, 20 µL injection volume, gold electrode working electrode, and 1V electric potential. The measurements were carried out using four different solutions for the mobile phase: 0.1 mol/L NaOH, 0.1 mol/L NaOH/0.1 mol/L CH3 COONa=1/1, 0.3 mol/L NaOH/0.1 mol/L CH3 COONa=1/1, and 0.3 mol/L NaOH/0.3 mol/L CH3 COONa=1/1. Two ion-exchange columns with a core-shell type filler reacted with an amine (S-30/70=St (styrene)/DVB (divinylbenzene)-5TMDAH (tetramethyldiaminohexane) and S-40/60=St/DVB-5TMA, φ4.6 mm × 150 mm) were used. The structure of core-shell column is shown in scheme 1.
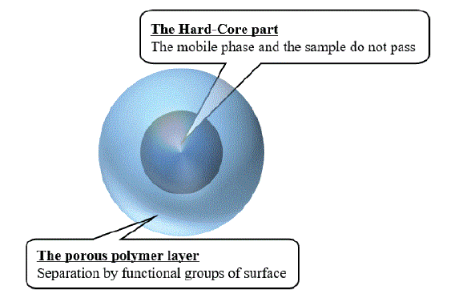
Scheme 1: Structure of the column with a core-shell filler
The calibration curves were prepared from the results with the mobile phase of 0.1 mol/L NaOH/0.1 mol/L CH3 COONa=1/1. The linearity was evaluated by the correlation coefficients of the prepared calibration curves. The repeatability, precision, and accuracy were evaluated by calculation of the RSD. The obtained calibration curves were used to calculate the LOD and LOQ from the following equations.
LOD=3.3 × (s⁄a) (eq. 1)
LOQ=10 × (s⁄a) (eq. 2)
s: SD of the intercept for calibration curve.
a: slope of the calibration curve.
Using 0.1 mol/L NaOH as the mobile phase, peaks for αCD, βCD, and γCD were not observed with neither S-30/70=St/DVB-5TMDAH nor S-40/60=St/DVB-5TMA columns (Figure 2).
Figure 2: Chromatograms of (a) αCD, (b) βCD, and (c) γCD
Mobile phase: 0.1 mol/L NaOH
Column type: (Ⅰ) S-30 / 70 = St / DVB-5TMDAH
(Ⅱ) S-40 / 60 = St / DVB-5TMA
Using 0.1 mol/L NaOH/0.1 mol/L CH3 COONa=1/1 as the mobile phase, peaks for αCD and βCD were not observed with either S-30/70=St/DVB-5TMDAH or S-40/60=St/DVB-5TMA columns (Figure 3). However, peaks for γCD were observed at retention times of over 60 min with an S-30/70=St/DVB-5TMDAH column and 27.9 min with an S-40/60=St/DVB-5TMA column (Figure 3c-I,II).
Figure 3: Chromatograms of (a) αCD, (b) βCD, and (c) γCD
Mobile phase: 0.1 mol/L NaOH / 0.1 mol/L CH3 COONa = 1/1
Column type: (Ⅰ) S-30 / 70 = St / DVB-5TMDAH
(Ⅱ) S-40 / 60 = St / DVB-5TMA
Using 0.3 mol/L NaOH/0.1 mol/L CH3 COONa=1/1 as the mobile phase, peaks for βCD and γCD were observed at retention times of 34.3 and 27.9 min, respectively, with an S-30/70=St/DVB-5TMDAH column. Peaks for αCD, βCD, and γCD were observed at retention times of 14.8, 16.0, and 12.0 min, respectively, with an S-40/60=St/ DVB-5TMA column (Figure 4). However, the specific peak for αCD was not observed with an S-30/70=St/DVB-5TMDAH column (Figure 4a-I).
Figure 4: Chromatograms of (a) αCD, (b) βCD, and (c) γCD
Mobile phase: 0.3 mol/L NaOH / 0.1 mol/L CH3 COONa = 1/1
Column type: (Ⅰ) S-30 / 70 = St / DVB-5TMDAH
(Ⅱ) S-40 / 60 = St / DVB-5TMA
Using 0.3 mol/L NaOH/0.3 mol/L CH3 COONa=1/1 as the mobile phase, peaks for αCD, βCD, and γCD were observed at retention times of 26.7, 34.1, and 26.7 min, respectively, with an S-30/70=St/ DVB-5TMDAH column. Peaks for αCD, βCD, and γCD were also observed at retention times of 6.5, 12.9, and 10.4 min, respectively, with an S-40/60=St/DVB-5TMA column. The retention time of each CD using an S-40/60=St/DVB-5TMA column was faster compared with an S-30/70=St/DVB-5TMDAH column (Figure 5). Incidentally, the measurements were investigated at 40°C column temperature (data was not shown). The sharp peaks were observed in the results of the measurement at 50°C column temperature compared to the results at 40°C column temperature. Therefore, the result of the column temperature 50°C was reflected.
Figure 5: Chromatograms of (a) αCD, (b) βCD, and (c) γCD
Mobile phase: 0.3 mol/L NaOH / 0.3 mol/L CH3 COONa = 1/1
Column type: (Ⅰ) S-30 / 70 = St / DVB-5TMDAH
(Ⅱ) S-40 / 60 = St / DVB-5TMA
Linearity, accuracy, repeatability, and precision: To evaluate the linearity of the calibration curve for each CD when 0.3 mol/L NaOH/0.3 mol/L CH3 COONa=1/1 was used for the mobile phase with an S-40/60=St/DVB-5TMA column, the correlation coefficient (R2) of the calibration curves of αCD, βCD, and γCD were calculated (Table 1).
| Samples | Regression equation |
Range (µg/mL) | Correlation coefficient |
| αCD | y=4777x+9549 | 5–100 | 0.999 |
| βCD | y=3147x+36254 | 25–500 | 0.999 |
| γCD | y=3857x–2559 | 25–500 | 0.998 |
Table 1: Parameters of calibration curves for αCD, βCD, and γCD (n=10)
Mobile phase: 0.3 mol/L NaOH/0.3 mol/L CH3 COONa=1/1
Column type: S-40/60-St/DVB-5TMA
The correlation coefficients of the calibration curves for αCD were greater than 0.999% at 5-100 µg/mL, and the correlation coefficients for βCD and γCD were greater than 0.998 at 25-500 µg/mL. The recoveries of α, β, and γCD were 100 ± 5%, and the relative standard deviation (RSD) were within 5% (Table 2).
| Samples | Resolution1) | Recovery (%) | RSD (%) |
| αCD | 1.7 | 96.90 ± 2.36 | 2.44 |
| βCD | 2.5 | 102.58 ± 3.74 | 3.65 |
| γCD | 3.5 | 96.49 ± 3.41 | 3.53 |
Table 2: Validation of the methods (n=10)
1)The injection peak (the retention time of peak top at 4.5 min) vs αCD, βCD and γCD (the retention time of each peak top at 6.5, 12.9 and 10.4 min)
Detection limit and quantitation limit: The limit of detection (LOD) and limit of quantification (LOQ) of each CD was calculated from the prepared calibration curves. The LOD and LOQ of αCD were determined to be 0.26 and 0.81 µg/mL, those of βCD were 7.65 and 23.19 µg/mL, and those of γCD were 3.87 and 11.74 µg/mL, respectively (Table 3).
| Samples | LOD (µg/mL) | LOQ (µg/mL) |
| αCD | 0.26 | 0.81 |
| βCD | 7.65 | 23.19 |
| γCD | 3.87 | 11.74 |
Table 3: Limits of detection and limits of quantification
s: SD of the intercept for calibration curve
a: slope of the calibration curve
A peak for γCD was observed by adding 0.1 mol/L CH3 COONa to 0.1 mol/L NaOH for the mobile phase. The adsorption of samples to the column has been reported to be suppressed by the addition of CH3 COONa to NaOH [10]. The adsorption of γCD to the column was suppressed and the elution time was shortened by the addition of 0.1 mol/L CH3 COONa. In addition, since the specific peaks for αCD and βCD were not observed, the dissociation of the alcoholic hydroxyl groups of αCD and βCD with 0.1 mol/L NaOH in the mobile phase was inferred to be insufficient. γCD as a cyclic oligosaccharide has more glucose units compared to αCD and βCD (the number of each glucose unit for αCD, βCD and γCD is 6, 7 and 8). Therefore, this result was suggested that γCD has more alcoholic hydroxyl group reacted by 0.1 mol/L NaOH as mobile phase compared to αCD and βCD.
The dissociation of the alcoholic hydroxyl group of CDs by a mobile phase containing 0.3 mol/L NaOH was determined to be sufficient because the specific peaks for αCD, βCD, and γCD were observed using a mobile phase of 0.3 mol/L NaOH/0.1 mol/L CH3 COONa=1/1 with anS-40/60=St/DVB-5TMA column. The peak for αCD was not observed with an S-30/70=St/DVB-5TMDAH column. It was inferred that the elution of αCD was suppressed due to its absorption onto the S-30/70=St/DVB-5TMDAH column.
Peaks for αCD, βCD, and γCD were observed for both columns using 0.3 mol/L NaOH/0.3 mol/L CH3 COONa=1/1 for the mobile phase. The absorption of the CDs to the column was suppressed more for the S-40/60=St/DVB-5TMA column since the result was faster compared with the S-30/70=St/DVB-5TMDAH column. Therefore, using an S-40/60=St/DVB-5TMA column and a mobile phase of 0.3 mol/L NaOH/0.3 mol/L CH3 COONa=1/1 was the best combination for the analysis of αCD, βCD, and γCD.
Since the correlation coefficients of αCD, βCD, and γCD were all greater than 0.998, the recovery was 100 ± 5%, and the RSD was <5%, the linearity, accuracy, and repeatability of this method were good and the precision was acceptable. In addition, the LOQ (0.81, 23.19, and 11.74 µg/mL) of αCD, βCD, and γCD under these measurement conditions were short compared with the LOQ using RI (about 200 µg/mL) or ELSD (about 50 mg/mL) detectors [2,6]. Thus, this new quantitative method using an ECD and a core-shell column is advantageous for the measurement of αCD, βCD, and γCD.
In this manuscript reported simple and efficient method for quantitative analysis of αCD, βCD, and γCD using core-shell columns. As a result, retention times of βCD and γCD were about the same. Therefore, it is necessary to accumulate investigation of columns and mobile phases to measure the mixture of αCD, βCD, and γCD simultaneously, and it is a future study topic. In addition, it is necessary an appropriate salt to obtain suitable detectability for each CD. In this study, the absorbance of each CD to core-shell column was inferred suppress by using 0.3 mol/L CH3 COONa as a mobile phase. In the future, the similar investigation was considered necessary when to study for other polymer formulations.
The measurement of CDs was made possible by using a sodium hydroxide solution as the mobile phase and a column containing coreshell type filler reacted with an amine. CDs could also be detected using a mobile phase of 0.3 mol/L NaOH/0.3 mol/L CH3 COONa=1/1. Sufficient linearity, precision, and accuracy were obtained by using an S-40/60=St/DVB-5TMA column. Thus, the quantitative analysis for CDs with an ECD and a column with a core-shell type filler was useful for the convenient analysis of αCD, βCD, and γCD. In future, this quantitative method will be useful method for a study of inclusion complex with CD.
The authors wish to thank DKK-TOA Co., Ltd., for their helpful advice regarding the ECD measurements, and to thank Cyclo Chem Co., Ltd. for the provision of αCD, βCD, and γCD.
The authors declare no conflicts of interest.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Mitsumori A, Narumi S, Inoue Y, Murata I, Mitomo S, et al. (2018) Quantitative Analysis of Cyclodextrins by Electrochemical Detector and Column with Core-Shell Type Filler. J Drug Res Dev 4(2): dx.doi.org/10.16966/2470-1009.141
Copyright: © 2018 Mitsumori A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access