Introduction
Adaption of a newborn infant to extrauterine life depends on many parameters including an intact development of the kidneys [1]. Normally, formation of nephrons concludes short before birth. However, when an infant is born preterm, the kidneys are still in a process of active nephrogenesis [2]. Autopsied preterm kidneys and a baboon model of preterm birth indicate that nephrogenesis can continue principally for up to 3 weeks in extrauterine life [3]. There is increasing evidence that preterm delivery is interfering the process of nephrogenesis causing in turn oligonephropathy estimated to be between 8 and 24% [4]. Pathologic data further reveal that in neonatal kidneys up to 18% morphologically abnormal glomeruli are present, which show a dilated Bowman’s space and a shrunken glomerular tuft. Occurrence of such glomeruli is restricted to the outer renal cortex pointing out that the nephrogenic zone is affected.
A therapeutic concept for preterm infants is to compensate harming influences by medication, to stimulate morphogenic activity within the nephrogenic zone and to prolong nephrogenesis during early postnatal development [5]. However, current data shows that such an approach must be adapted to peculiar structural and functional features within the nephrogenic zone [6]. Equally important, there is an urgent need of investigations dealing with the synthesis, secretion and concrete transport of morphogens locally involved in the process of nephrogenesis [7]. Finally, data for application of drugs, which exhibit promising options to prolong nephrogenesis, are missing up to date
Reliable Perspective for Microscopic Analysis
During fetal growth of a mammalian kidney the nephrogenic zone is restricted to the cortex cortices of parenchyma [8]. For microscopic analysis randomly cut sections do not help. To obtain comparable perspectives, a monopapillary kidney of mice, rat or rabbit is divided in the middle between both poles for histological preparations. A human fetal kidney is cut best from the capsule to the papilla of a lobus. Following this advice, the section plane shows parenchyma in the cortex cortices that is orientated along the lumen of collecting duct (CD) tubules (Figure1). Due to incomplete histological preservation, pathological specimens of human kidney are often hard to interpret. To overcome some of the barriers, the nephrogenic zone of neonatal rabbit fixed under controlled conditions serves here for illustration.
Constant Position of the Nephrogenic Zone
The nephrogenic zone extends as a band in the cortex cortices of a fetal kidney and shows a width between 60 and 100 µm depending on species [9]. Its outer aspect is covered by the organ capsule, while the inner side is facing maturing CD tubules and developing nephrons including first stages of arising glomeruli (Figure 1). Located between these two limits, the entire cell biological machinery is contained here to maintain cell stemness, induction and initial development of nephrons. Concrete components of the nephrogenic zone recognized by optical microscopy are ureteric bud-derived CD ampullae adjoining each other, juxtaposed metanephric mesenchyme, renal vesicles and S-shaped bodies. Sections stained by hematoxylin-eosin solution show the nephrogenic zone as a ‘blue strip’ [10].
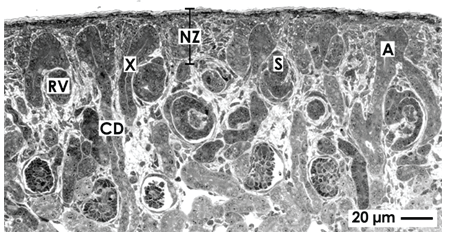
Figure 1. Optical microscopy of the nephrogenic zone (NZ) localized in the cortex cortices of a neonatal rabbit kidney. The histological section lines perpendicular to the organ capsule and in parallel to lining collecting duct (CD) tubules. They exhibit a neck (X) and form at their endings a CD ampulla (A). At the lateral aspect of a CD ampulla renal vesicles (RV) and S-shaped bodies (S) indicate active nephrogenesis.
Incomplete Vascular Supply
The incomplete vascular supply is a unique feature of the nephrogenic zone. Earlier performed histochemistry with Ulex europaeus I lectin on human fetal kidneys informs that in the area of starting nephrons not intact capillaries but only spreading endothelial cell strands exist [11]. In neonatal rabbit kidney it was recorded by immunohistochemistry that capillaries line from cortical radiate arteries towards the cortex cortices. Forming vessels are recognized on developing glomeruli. Strands of endothelial cells line to the lower cleft of S-shaped bodies, where the glomerular tuft is arising (Figure 2). Some endothelial cells occur at the lateral aspect of CD ampullae [12]. However, at the niche site including the tip of a CD ampulla and neighboring mesenchymal stem/progenitor cells, endothelial cells are not present.
Expression of endothelial nitric oxide synthase was found in the kidney of rat on developing S-shaped bodies, but not within the mesenchymal cell layers [13]. In homology to the ureteric bud, it appears most probable that Wingless Int-1 (Wnt7b) protein expressed by epithelial stem cells at the lateral aspect of CD ampullae activates canonical Wnt signaling in surrounding interstitial cells to establish capillaries [14]. An actual investigation on developing mice kidney exhibits that forming vessels at the nephrogenic zone remain un-perfused, although oxygenation is able to drive nephron progenitor differentiation [15,16]. Further capillaries within the capsule produce interstitial fluid to transport it in a complex tunnel system (Figure 2) [8].
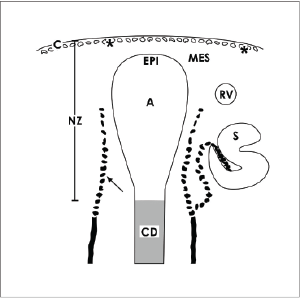
Figure 2. Schematic illustration informs about the incomplete vascular supply of the nephrogenic zone (NZ) in neonatal rabbit kidney. Label by antibody EC1 [12] depicts that endothelial cells arise from cortical radiate arteries (arrow) lining in parallel to collecting duct (CD) tubules. Endothelial cells migrate to the lateral aspect of an ureteric bud derived CD ampulla (A) and to the lower cleft of a S-shaped body (S). In addition, cells of the tunica muscularis within the organ capsule (C) form intracellular and extracellular tunnels (asterisks) [8]. It is obvious that the area of the niche including the tip of a CD ampulla with epithelial (EPI) cells and neighboring mesenchymal cells (MES) is avascular.
Coordinates within the Nephrogenic Zone
The outer limit of the nephrogenic zone is the organ capsule (Figures 1 and 3a). In neonatal rabbit kidney it consists of a tunica fibrosa and a tunica muscularis including atypical smooth muscle cells [8]. Beyond the capsule only 2 layers of metanephric mesenchymal (MES) stem cells occur. Below and separated by a striking interface, the tip of ureteric bud-derived CD ampullae containing epithelial (EPI) stem cells is present. Their tips have a distance of 15 µm to the inner side of the organ capsule. All of CD ampullae have the same orientation. At their lateral aspects condensed mesenchyme, renal vesicles and S-shaped bodies occur reflecting morphological signs of active nephrogenesis. The dilated part of a CD ampulla continues to a neck and then to a shaft connected with a differentiating CD tubule [17]. This site is the exact inner limit of the nephrogenic zone.
Perimeters of the Renal Stem/Progenitor Cell Niche
The nephrogenic zone is homing different kinds of stem cells (Figure 3a). Mesenchymal stem cells occur as well in the organ capsule [18] as in the underlying metanephric mesenchyme [19]. Epithelial stem cells are integrated in the tip of a CD ampulla [20]. Consequently, as well parenchyma as stroma are developing from this pool of cells. In a wider definition, as well the capsule as mesenchymal and epithelial stem cells of the nephrogenic zone represent the renal stem cell niche. In a narrower sense, only the tip of a CD ampulla containing epithelial stem/ progenitor cells and some nephrogenic (Six2+/CITED1+/Bcl2+) cells in the neighboring cap mesenchyme (CM) are regarded as a niche [21-23]. This latter definition points to the site, where induction and initial formation of a nephron take place.
Fastening of the Niche and Positioning of Stem Cells
Development of renal parenchyma is driven by a process, which is called branching morphogenesis [24,25]. In a human kidney the invading ureteric bud produces first the tubule system in the medulla and then collecting duct (CD) tubules in the cortex. During radial extension they show a special branching pattern in the inner and then in the outer cortex [26]. In the nephrogenic zone of neonatal rabbit and before birth in a human kidney this spatiotemporal program raises in elongating CD tubules bifid branches. Its end is orientated towards the covering mesenchyme. Since a branch shows a dilated form, it was specified CD ampulla.
For the human kidney data are lacking, but in neonatal rabbit kidney exact encountering of epithelial and mesenchymal stem cells is supported by microfibers that link the inner side of the organ capsule with the tip of a CD ampulla (Figure 3b). In the basal lamina at the tip of a CD ampulla laminin ɣ1 and the proteoglycan agrin are contained [8]. Here microfibers labeled by anti-collagen type I, II, III respectively Soybean agglutinin (SBA) originate, span through the 2 layers of mesenchymal stem cells to be linked on the inner side of the organ capsule. The mounting demonstrates that epithelial and mesenchymal stem cells do not meet by accident, but are integral part of a cage. It coordinates positioning at the place but keeps contained cells by a short lead close to the capsule.
Exact movement within an individual niche is adjusted by the secreted Bone morphogenic protein (BMP) antagonist Cerberus homologue1 (Cer1), Ret protooncogene (Ret) and ETS translocation variant4 (Etv4) [20,27]. By positioning the tip of a CD ampulla, mesenchymal cells come in close neighborhood. While approaching, some of mesenchymal cells acquire competence to respond to morphogens. This operation is under control of protocadherin (cadherin family member14: FAT4/ dachsous cadherin related1: Dchs1) signaling [28]. Yet epithelial stem/ progenitor cells in the tip of a CD ampulla are faced by GDNF+/Six2+/CITED1+mesenchymal cells [22,23]. When the exchange of morphogens was successful, few mesenchymal cells separate, aggregate and perform a mesenchyme-to-epithelial transition (MET) to develop into a renal vesicle at the lateral aspect of the related CD ampulla. For remaining mesenchymal cells the transcription factor Zeb1 is controlling the balance between proliferation, migration and apoptosis [29].
When impairment of nephrogenesis in the kidney of preterm infants is under debate, remodeling of illustrated microfibers appears to be important (Figure 3b). This process requires tissue transglutaminases (TGases), matrix metalloproteinases (MMPs) and membrane targeted MMPs (MT-MMPs) controlling synthesis and degradation of extracellular matrix [30-32]. An equilibrated activity of those TGases controls also growth factor-stimulated signaling and in turn cell proliferation [33,34]. Whether the balance of TGase activity on microfibers or on the basal lamina of a CD ampulla tip is disturbed and intrinsic activity within renal stem cell niches of preterm infants is impaired thereby, waits for investigation. However, when an increased activity is detected, therapeutic application of inhibitors of TGases may help to find back to a consummate equilibrium between synthesis and degradation [35].
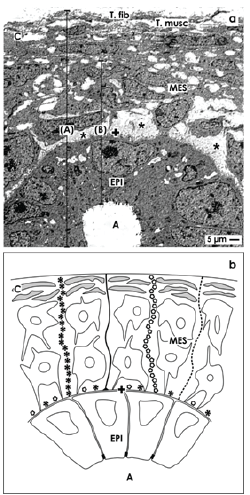
Figure 3. Details of the nephrogenic zone in neonatal rabbit kidney shown by (a) transmission electron microscopy and (b) schema. The capsule (C) consists of a tunica fibrosa (T.fib) and tunica muscularis (T. musc). Epithelial (EPI) stem/progenitor cells are enclosed the tip of a CD ampulla (A) and are covered by a basal lamina (+). Mesenchymal (MES) and epithelial (EPI) stem/progenitor cell bodies are separated by a striking interface (asterisks). (A) In a wider definition both nephrogenic zone and the capsule form the renal stem cell niche. (B) In a narrower sense, only the tip of a CD ampulla containing epithelial stem cells and some above positioned GDNF+Six2+mesenchymal cells are regarded as a niche. b) Schema shows microfibers linking a stem cell niche with the inner side of the capsule. Histochemistry exhibits that in the basal lamina of a CD ampulla tip laminin ɣ1 and agrin are contained. At this site microfibers binding Soybean Agglutinin (SBA; black line) and anti-collagen type I (black asterisks), type II (light circles), and type III (dotted line) originate, cross the interface, line through the group of mesenchymal stem cells to fasten on the capsule.
Interface between Mesenchymal and Epithelial Stem Cells
Epithelial and mesenchymal cell bodies within the niche do not touch but stand at a distance between 1 to 2 µm (Figures 3a and 4a) [36,37]. This special cell arrangement can be seen by optical microscopy [38-41], transmission electron microscopy [42-45] and it is present in mice, rat, rabbit and human kidney. Ultrastructural analysis exhibits that a basal lamina covers the tip of a CD ampulla. Its lamina fibroreticularis consists of a noticeable fibrillar meshwork [46]. Further projections (also called cytonemes, signaling filopodia, protrusions) of mesenchymal cells cross the interface to contact the basal lamina (Figures 3a and 4a). The interface looks blank, when conventional fixation by glutaraldehyde (GA) solution for transmission electron microscopy takes place. Numerous braces of proteoglycans on the surface of projections and on the basal lamina become visible, when fixation of specimens is performed by GA solution including cupromeronic blue (Figure 4b) [47]. Application of GA solution including ruthenium red (Figure 4c) or tannic acid (Figure 4d) unmasks further filigree extracellular matrix at the interface [37]. One can imagine that any imbalance in synthesis or degradation of extracellular matrix within the renal niche will impair nephrogenesis.
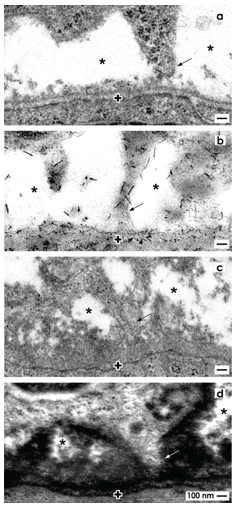
Figure 4. Transmission electron microscopy depicts mesenchymal cell projections (arrow) contacting epithelial cells and unveils extracellular matrix at the interface of the renal niche. a) Specimens fixed by conventional glutaraldehyde (GA) solution suggest that the interface (asterisk) appears blankly. b) Fixation by glutaraldehyde solution including cupromeronic blue (CMB) shows that numerous braces of proteoglycans are recognized on the surface of cell projections and within the basal lamina (arrow heads) of epithelial stem cells. c) Specimens fixed by GA solution including ruthenium red (RR) or d) tannic acid (TA) unmasks textured extracellular matrix at the interface and on the basal lamina labeled by a cross (+).
Cell to Cell Communication
Although bodies of mesenchymal and epithelial stem cells are separated by an interface, projections of mesenchymal cells cross it, to establish a contact at the tip of a CD ampulla (Figures 3a and 4a) [48]. At this site integrin α8β1 is localized, which binds to nephronectin on the basal lamina covering epithelial stem cells [49-51]. Also the micro-tubuledependent motor protein kinesin (KIF26B) occurs here, which regulates cell attraction, signal transduction and developmental patterning [52-54].
Actual data illustrate that a mesenchymal cell projection penetrates the basal lamina of epithelial stem cells. Its final passage is surrounded by extracellular matrix forming a special sleeve [48]. Within the end of a projection, in the approaching zone at the basal lamina and in the basal plasma membrane of an epithelial stem/progenitor cell tunneling nanotubes are integrated (Figure 5). This important finding illustrates an intercellular path between mesenchymal and epithelial cells that is optimally suited for cell to cell communication including the transport of a variety of molecules [55]. While in transmission electron microscopy the moment of an actual physiological status is frozen, actual time laps imaging shows that mesenchymal cells are motile so that they attach and detach from the CD ampulla tip across time [56]. One can imagine that any disturbance of communication between mesenchymal and epithelial stem cells including restricted functionality in cell projections and/or tunneling nanotubes will impede nephron induction leading in turn to impairment of nephrogenesis.
Pleiotropic Actions of Morphogens
Stem cells within the renal niche are under control of different morphogens. They have pleiotropic tasks such as triggering of stemness, cell proliferation, competence, induction and initial formation of nephrons [7]. In a human kidney this machinery is active from begin of organ anlage up to birth, while in other mammalian species nephrogenesis proceeds during the early postnatal period [5].
An upstream function by supervising survival, stemness and proliferation of stem cells fulfills for example morphogen Mouse double minute 2 homolog (Mdm2). Its successful signaling results in expression of typical site-specific markers such as Amphiphysin, Cited1, Sall1 and Pax2 [57].
A central role have morphogens, which operate competence, induction and subsequent nephron formation. This process starts, when an elongating CD tubule divides into bifid branches to form CD ampullae [58]. In turn, on neighboring mesenchymal cells the time slot for competence opens. For example, BMP7 makes possible to respond to an inductive signal evocated by a morphogen [59]. In that special case only GDNF+/Six2+/CITED1+ mesenchymal cells will respond to epithelial cells contained in the tip of a CD ampulla [22,23]. Downregulation of Six2 for example by knockdown of neurofibromin with small interfering RNAs results in loss of competence and an increased ratio of apoptotic cells [60].
In a next step morphogens induce initial formation of a nephron [61,62]. For the renal niche it comprises a reciprocal signaling between mesenchymal and epithelial stem cells. Involved are Glial cell line-Derived Neurotrophic Factor (GDNF), Bone morphogenetic proteins (BMP4, BMP7), Wnt family members (Wnt4, Wnt5a, Wnt9b) and Fibroblast growth factor (FGF8) [63-68]. Transmission of these signals is successful, when receptors such as FGFr1, FGFr2, Gfra1, Notch 2, Ret tyrosine kinase receptor and transcription factors such as BRN1, FoxC2, Osr1, Sall1, Pax2 and Wt1 are activated [7].
After signaling of morphogens some GDNF+/Six2+/CITED1+ mesenchymal cells separate and then aggregate on the lateral side of a related CD ampulla [22,23]. Here they perform a mesenchyme-toepithelial transition (MET) to develop into a renal vesicle as the first visible sign of a developing nephron [69].
Each Morphogen must reach its Target
For years it was believed that epithelial and mesenchymal stem cells within the renal niche meet by chance and that transport of morphogens occurs by diffusion. Further it was supposed that the distance between involved cells can be neglected [58,7]. Under such ideal conditions a sharp gradient would arise, so that an effective concentration of a morphogen reaches its receptor [70]. All of that sounds convincing, but has not been investigated thoroughly for the niche.
Some hints about the transport of a morphogen deliver transfilter culture experiments. For example, NIH3T3 mouse embryo fibroblast cells expressing morphogen Wnt4 were cultured for tubule induction on the one side, while isolated nephrogenic mesenchyme was placed on the other side of a filter [71]. The result was that separating filters with pore sizes of 0.1 µm and above support induction including tubule formation, while pores of 0.05 µm abolish it. In any case, morphogens are so small that they can cross a pore of this diameter. Surprisingly, solubilized molecules in form of a supernatant from Wnt4 expressing cells were not able to induce formation of tubules. Thus, the transport of a morphogen during induction of a nephron does not depend alone on diffusion [72].
As well earlier morphological findings in niches of embryonic kidney [73] as additional transfilter culture experiments [74] contradict the general assumption that all morphogens are transported by diffusion between mesenchymal and epithelial cells. Actual morphological indications for a sophisticated transport of morphogens are the spatial separation of mesenchymal and epithelial cell bodies (Figure 3a), a striking interface filled with filigree extracellular matrix and a basal lamina covering epithelial cells (Figures 4b-4d) [6,48]. Such an environment impedes diffusion and enables selective binding of morphogens during their transport on extracellular matrix [75]. Communication between mesenchymal and epithelial cells via projections including tunneling nanotubes points out that a route for a dosed transport of various molecules exists (Figure 5) [76].
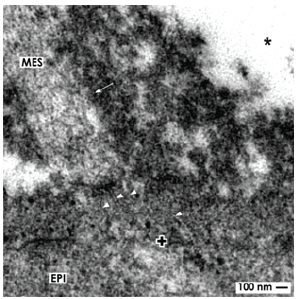
Figure 5. Transmission electron microscopy shows cell to cell communication in the renal stem cell niche. A projection (arrow) of a mesenchymal cell (MES) is crossing the interface to establish a contact with epithelial (EPI) cells via tunneling nanotubes (arrow heads).The basal lamina of epithelial cells within a CD ampulla is marked by a cross (+).
A biophysical point of view for a sophisticated transport of morphogens is that each group of them has different molecular properties. They can be sorted according to good [22,63,77], minor [78,79] and poor [80-82] solubility in saline solution [83]. This again appears as a mirror for a good, minor and poor transport of morphogens in interstitial fluid by diffusion. When such a sorting is performed (Figure 6), it is possible to allocate the transport of involved morphogens to recently detected morphological findings (Figures 3a, 4 and 5). Since concrete data for the human renal stem cell niche are not available, the here proposed concept is based on morphological findings raised in neonatal rabbit kidney and data collected from other developmental systems [84,85].
Morphogens meet Ultrastructure
Fixation of specimens with glutaraldehyde (GA) solution including cupromeronic blue, ruthenium red or tannic acid for transmission electron microscopy demonstrates filigree extracellular matrix at the interface of the renal stem/progenitor cell niche (Figures 4b-4d) [83,84]. A complementary but very little space does not show any label, appears to contain only interstitial fluid and is consequently suited for diffusion of molecules (Figure 6.1). A candidate for a here transmitted morphogen is GDNF that is built up by 134 amino acids, secreted as a glycoprotein and therefore well soluble in interstitial fluid [86]. Surprisingly, only GDNF synthesized by mesenchymal stem cells was up to date defined as a longdistance diffusible morphogen that binds on Ret tyrosine kinase receptor and a co-receptor GFRα1 localized at the tip of a CD ampulla [87,22].
Label of cupromeronic blue on mesenchymal cell projections and on the basal lamina covering epithelial stem/progenitor cells illustrates syndecans and/or glypicans, while label by ruthenium red or tannic acid points to perlecans and other proteins of extracellular matrix [88]. Literature informs that especially proteoglycans exhibit a high affinity for morphogens and are able to modulate kidney development by interacting with GDNF, molecules of the FGF and TGFβ superfamilies, EGF receptor ligands and HGF [89-92]. The general concept is that binding of individual morphogens on proteoglycans can be seen as a ‘morphogenic switch’ that influences as an inhibitor or facilitator the fine-tuning of a morphogen gradient (Figure 6.2). For example, environment lacking heparan sulfate proteoglycans (HSPGs) does not support formation of an effective Wnt gradient abolishing in turn further development [88].
Morphogens such as Wnt4, Wnt5a and Wnt9b have a distinct influence on renewal and differentiation of nephron progenitor cells, CD ampulla branching and nephron induction [93,7]. Moreover, Wnt molecules have post-translational modifications in form of a saturated palmitic acid and an unsaturated palmitoleic acid resulting in a poor solubility within interstitial fluid that impedes diffusion from one cell to the other [81]. There is some evidence that Wnt molecules are not secreted into interstitial fluid for further diffusion but are deposited by epithelial cells near contacting mesenchymal cell projections. By this mechanism they can reach the plasma membrane of the target cell to find a directed transport [94,95]. Tkv-GFP receptor puncta of cell projections in Drosophila were shown to move here either in an anterograde or retrograde direction (Figure 6.3) [96].
Morphogen Sonic hedgehog (Shh) controls renal patterning [97,98]. It is not secreted into the interstitium, but is produced in form of a particle that remains associated during transport with the surface on cell projections (Figure 6.3) [99,100].
Bone morphogenic proteins (BMPs) belong to morphogens with poor solubility in interstitial fluid [22]. For this reason the transport of a BMP molecule from one cell to the other by diffusion in the interstitial fluid is unlikely. Instead, transport of BMPs at the contact site between a mesenchymal cell projection and an epithelial cell appears more probable (Figure 6.3). Here a BMP molecule can bind on the plasma membrane for transport on its receptor as it was demonstrated for Drosophila Tkv [101,102].
Tunneling Nanotubes enable Transport at the Right Site
and Time
Actual morphological data exhibit that projections of mesenchymal stem cells cross the interface to contact the basal aspect of epithelia cells (Figures 3a and 4) [83,84]. In addition, between the end of a mesenchymal cell projection and the basal plasma membrane of an epithelial cell tunneling nanotubes establish a functional cell to cell communication (Figures 5 and 6.3). Occurrence of tunneling nanotubes shows that within the renal niche an up to date not considered intercellular transport system for organelles, membrane compounds and other kinds of molecules exists [103-108].
Transport functions of tunneling nanotubes were investigated till now not within the niche but by cultured renal cells [55,109]. Precise ligand distribution via cell to cell contacts and signaling filopodia was demonstrated for BMP2 and Wnt morphogens [110,111]. Although yet not examined, it appears most likely that morphogens are transported in the renal niche this way at the right site, time and in adequate concentration to control stemness and to trigger nephron induction (Figure 6.3) [83,84,112].
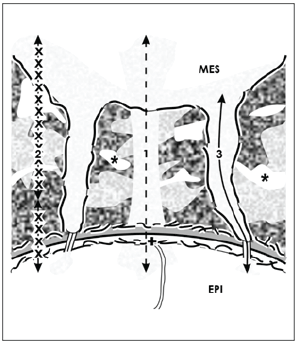
Figure 6. Schematic illustration informs about 3 possible routes for the transport of morphogens within the renal stem cell niche. Mesenchymal (MES) and epithelial (EPI) cells are separated by an interface (asterisk) including textured extracellular matrix. Projections of mesenchymal cells cross it to establish a cell to cell communication via tunneling nanotubes. On this situation it is speculated that morphogens with good solubility are transported by diffusion (1). Morphogens with minor solubility are secreted in interstitial fluid and then bound on extracellular matrix, where they are delivered on demand (2). Morphogens with poor solubility are transported in cell projections and tunneling nanotubes (3). The basal lamina of epithelial stem cells is marked by a cross (+).
Shuttle by Extracellular Vesicles
Beside mentioned routes, the transport of morphogens between
cells may also take place by vesicles such as exosomes (40-100 nm) or
microvesicles (100-1000 nm) [113-115]. In such particles as well mRNA,
microRNA as synthesized morphogens principally can be shuttled [116-
118]. Although demonstrated for regenerative processes in the kidney,
with exception of morphogen Shh literature is lacking that informs about
transport of morphogens by vesicles within the nephrogenic zone.
Regenerative Medicine encounters the Nephrogenic Zone
Actual literature informs that quite different influences can cause impairment of nephrogenesis in preterm infants [119]. Independent from chemical nature all of them converge finally in the nephrogenic zone and the here contained renal stem cell niches. It is the definitive scene, where maintenance of stemness, positioning of cells and initial development of nephrons are controlled. Considerations about a concept for preterm infants prolonging nephrogenesis lead inevitably to the peculiar microanatomy of the nephrogenic zone and the integral functions of involved morphogens. In that regard one must keep in mind that morphogens are on the one hand highly effective molecules triggering developmental processes. On the other hand, their therapeutic use might be associated with up to date hidden biomedical risks. For example, exogenously applied GDNF promotes formation of unwished ectopic ureteric buds [120].
The bodies of mesenchymal and epithelial stem cells, their spatial separation by a striking interface, peculiar extracellular matrix and communication via mesenchymal cell projections including tunneling nanotubes must be seen as a structural-functional ensemble (Figures 3a and 4) [83,84], which is controlled by a set of morphogens with quite different biophysical features [7]. The actual problem is that about stimulation/inhibition of local morphogen synthesis, secretion and binding on extracellular matrix during transport only little concrete information is available. Paraphrased, a site-specific therapy for the nephrogenic zone including the niches for prolongation of nephrogenesis in preterm infants needs first answers to unsolved issues and then a critical selection of eligible drugs. Imaginable is either administration of a drug that stimulates the synthesis of genuine morphogens within the nephrogenic zone or application of pharmacologically active morphogens by a smart drug delivery system.
Drug Delivery is the Crucial Point
Due to an incomplete vascular supply the nephrogenic zone is a bradytroph district. In the specific case the area of the actual stem cell niche is avascular (Figure 2) [12]. This fact is an obstacle for a local drug delivery prolonging nephrogenesis. Probably it will be possible to transport drugs via cortical radiate arteries and/or via the rete capsularis including the tunnel system enabling diffusion in interstitial fluid from two directions towards the nephrogenic zone [8].
Morphogens such as GDNF or FGF8 with a good solubility in saline have a chance to reach the niches by diffusion (Figure 6.1). For morphogens such as BMP4 or BMP7 a therapeutic administration is complicated. After secretion BMPs are transported by restricted diffusion and interaction with extracellular matrix (Figure 6.2). Thus, not by the therapeutic administration, but by the local extracellular matrix within the nephrogenic zone is decided upon their free accessibility to the target. For morphogens such as Wnt4, Wnt5a, Wnt9b or Shh an administration becomes unpredictable, since they are not soluble in saline solution, can bind optionally on extracellular matrix and on cell projections [71]. Transport of mentioned morphogens is also thinkable along the plasma membrane and via tunneling nanotubes (Figure 6.3) [121-123]. Possibly, isolation and culture of the nephrogenic zone in its original composition will help in a first step to solve some of the mentioned problems [124].
Conclusion
Preterm infants are suffering frequently on impaired nephrogenesis causing in turn oligonephropathy with lifelong disease risks. Consequently, a pharmacological concept prolonging nephrogenesis in preterm infants is required. However, due to incomplete vascular supply, complex microanatomy and diverse signaling of morphogens a site-specific drug delivery in the nephrogenic zone will be a challenge. Drugs will act, when they exhibit a good solubility to reach the target by long distance diffusion, while drugs with poor solubility will precipitate. However, administration and biophysical features of a drug are not the sole problem. Even critically must be analyzed its nephrotoxicity and possible side effects. Due to an unclear form of administration and unsure action on the target, it is questionable, whether a therapeutic concept for prolongation of nephrogenesis will be available in the next future.
Funding
The project was supported by the Emeriti Research Fund, University of Regensburg, D-93053 Regensburg, Germany







