
Table 1: Lists some examples of herbs that have a beneficial effect

Narayanan S1* Young DS2
1Department of Pathology and Laboratory Medicine, Weill Medical College of Cornell University, New York, USA*Corresponding author: Narayanan S, Department of Pathology and Laboratory Medicine, Weill Medical College of Cornell University, New York, USA; E-mail: shn2011@med.cornell.edu
Herbal medicine has been with us literally from the dawn of civilization. The Chinese treatise on herbal medicine “Shen Nong Ben Cao Jing” also known as the “The Herbal classic of the Divine Plowman” dates to 2700 BC. During 2500-1800 BC in India the medicinal properties of herbs were documented in the Hindu religious texts that became the basis of Ayurvedic medicine in India. In Europe dating to the time of the Roman emperor Marcus Aurelius in the 1st century A.D. herbs have been in use for their medicinal effects. It is instructive to note that some of the widely prescribed Western drugs have been derived from extracts of herbs from medicinal plants [1]. Thus the widely prescribed diabetic drug Metformin is derived from a plant called Galega officinalis. Similarly, the drug Digoxin is derived from digitalis. Likewise, Artemisinins used to treat malaria was isolated from the plant Artemisiae folium that, incidentally, was cited in the 2015 Nobel Prize. Many such examples of Western drugs derived from Herbs can be cited. It is well known that there are herbs that have beneficial effects including protecting the liver, lowering plasma cholesterol concentration, improving glycemic control, relieving stress and improving cognitive function to list a few. However, there are herbs, which have a toxic effect on the liver and kidney function. Herbs and natural products may influence the metabolism of drugs and thus affect therapy. Herbs and natural products may also influence the results of certain laboratory tests.
The use of Herbal medicines is so widespread worldwide that in the year 2000, the annual sales in the United States alone amounted to 4 billion dollars, while the sales in Europe were as high as $6.7 billion. When one adds the sales in Japan, China, India, Southeast Asia and the rest of the world, the dollar figure is staggering.
While drug interferences in laboratory testing have been widely reported and documented, only in recent years with the surge in the use of herbal medicines has their effect on laboratory tests become truly recognized leading to their effects being catalogued.
We will first address the mechanisms of herbal effects and point out both the beneficial and toxic effect of some herbs including the presence of contaminants frequently contained in some herbal medicines. We will also highlight with some examples the effect of herbs on laboratory tests and herb-drug interactions that may affect the diagnosis and treatment of patients.
While the beneficial effect of an herb is straightforward with some active principle in the herb promoting either liver, kidney, prostate or pancreatic function thus contributing to the health of the subject, it is the interaction of herbs with drugs that are of concern to the clinician and laboratory since this affects the diagnosis and treatment of patients.
Certain herbs can either induce or inhibit the cytochrome P450 (CYP) family of enzymes that are involved in the metabolism of drugs, thus affecting the therapeutic effect of these drugs. One major CYP isoform CYP3A4 present in the liver and intestine is involved in the metabolism of a majority of prescription drugs. Many other CYP isoforms are also involved in the metabolism of specific drugs, which we will refer to when we discuss specific herb-drug interactions. Several herbs can also affect the activity of the efflux transporter P-glycoprotein which is an ATP-dependent transport protein present in the kidney and the gut and thus affect the concentration of a drug which is a substrate for the efflux transporter, thereby affecting its absorption and clearance. Herbs, by affecting the uptake of a drug by specific transporters such as organic anion-transporting polypeptide A (OATAP1A2), can influence the concentration of a drug [1].
While several herbs affect the laboratory monitoring of drugs and thus affect therapeutic management, the reason that herb usage is so widespread is due to the salutary effects of some herbs. We will cite a few examples of herbs that are beneficial.
An herb that is hepatoprotective is milk thistle. Known by its botanical name Silybum marianum, an extract of milk thistle seeds contains a substance called Silymarin, which is a mixture of three isomers, one of which is Silibinin whose content is used to standardize preparations of milk thistle. In a study of 106 patients with alcoholic liver disease who were treated for four weeks with a milk thistle preparation, a striking reduction in liver enzyme activities in serum (aspartate aminotransferase (AST) of 57.5% and alanine aminotransferase (ALT): of 62.2% was observed [2].
An example of another herb that has beneficial properties is saw palmetto, which is used to treat urinary flow problems associated with benign prostatic hyperplasia (BPH). The herb derived from sabal fruit is known as Serenoa repens or Sabal serrulata. A large randomized controlled trial has described its benefits in patients with BPH [3].
The herb ginseng, which is widely applied in Chinese medicine, is used as a restorative tonic and to increase resistance to stress. It is also used to treat gastric disorders. The dry root of Asian ginseng, also known as Panax ginseng, contains several different ginsenosides or panaxosides. The active constituents in ginseng are triterpenoid saponin glycosides. The ginsenosides Ro, Rg1 and Rg2 and panaxynol have anti-platelet activity [4]. The glycans present in ginseng have a hypoglycemic effect apparently due to its effect in stimulating insulin secretion. In one study at a dose of 100 mg or 200 mg/day of ginseng administered to type 2 diabetic subjects for 8 weeks a statistically significant reduction of fasting blood glucose concentrations was achieved. A statistically significant decrease in the Hemoglobin A1C (HbA1C) was also noted when 200 mg/day of ginseng was administered for 8 weeks [5].
Fenugreek (Trigonella foenumgraceum) is an herb that has wide application in Ayurvedic medicine and is also a staple of Indian cooking. The ripe dried seeds of the plant have a hypoglycemic and antilipidemic effect. In one study a 5 mg/day dose of fenugreek administered to 20 mild Type 2 diabetes patients for 1 month resulted in an 18% reduction of the blood glucose concentration. In the same study a statistically significant reduction of serum cholesterol and triglyceride concentrations was noted at a dose of 5 mg/day for 3 months [6].
A mixture of 8 Chinese herbs that is widely used as a restorative tonic in Japanese (Kampo) Medicine is called Hachimi-jio-gan. It is also used to treat diabetes, prostate problems, erectile dysfunction and a variety of ailments that affect the elderly. In one study Hachimi-jio-gan administered to elderly subjects (age range 66-82years) for 7 months, causeda statistically significant increase in serum high density lipoprotein (HDL) concentration and a decrease in lipid peroxide levels [7] (Table 1).

Table 1: Lists some examples of herbs that have a beneficial effect
Some herbs are toxic to the liver or kidney. Examples of hepatotoxic herbs are Black cohosh, Chapparal and Germander.
Black cohosh known by its botanical name Cimicifuga racemosa is used to treat symptoms of the menopause and premenstrual syndrome. Upon using this herb to treat symptoms of menopause for one week, a 47-yearold woman had striking elevation of liver enzymes in serum (AST: 91-times the upper limit of the reference interval (ULRI) and ALT: 57- fold increase from the ULRI (ULRI of AST: 35 U/L and ALT: 40 U/L). The hepatotoxic effect was so severe that she required a liver transplant [8].
Chapparal (Larrea tridentate), which is also available in tea form, is used to treat a variety of conditions ranging from inducing weight loss to chronic skin disease. A review of 18 case reports noted an increase in liver enzyme activities (aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase), and bilirubin concentrations in users of this herb. 1-17 weeks after the discontinuance of the herb, the liver enzymes and bilirubin returned to within the normal reference interval [9].
The blossoms of germander (Teucrium chamaedrys) plant are used to treat obesity. However, seven cases of acute hepatitis have been reported 3-18 weeks after healthy subjects consumed the recommended dose of the herb (600-1,620 mg/day). 1.5-6 months after the discontinuation of the herb liver enzyme activities and bilirubin concentrations returned to within the normal reference interval [10].
An example of an herb that is nephrotoxic is Aristolochia Fang Chi, which is a component of a Chinese herbal preparation called Mu tong. The herb contains two acids, aristolochic acids I and II, which are nephrotoxic. The herb is used to treat eczema and is also used in some slimming pills. Two women who took this herb over a 2-6-year period to treat their eczema developed end-stage renal disease (creatinine: 7.5-9.5 mg/dL and urea nitrogen: 58-100 mg/dL) and became candidates for kidney transplantation [11] (Table 2).
These may be drugs added intentionally to boost their effects or heavy metals either added or present naturally in the plant due to their absorption from soil rich in such metals [1]. Lead, Arsenic, cadmium and mercury have crept in as contaminants in many Indian (Ayurvedic) and Chinese herbal medicines. Lead poisoning has resulted due to lead contamination from some Ayurvedic herbs [12]. Arsenic contamination present in some herb preparations of bladder wrack (Fucus vesiculosus) can lead to nephrotoxicity [13].
One of the most widely used herbal preparations in the United States, used to treat depression, and is St. John’s wort. Known by its botanical name Hypericum perforatum it is actually a mixture of several constituents, of which the major active component is hyperforin. This activates the human nuclear pregnane X-receptor (hPXR), which in turn activates the CYP3A gene in the liver and small intestine. By inducing the CYP3A4 isoform St. John’s wort causes a decrease in the concentration in blood of Theophylline, Indinavir, Ritonavir, Nelfinavir, Sirolimus, Tacrolimus, Amiodarone, Amitryptyline, Nortriptyline, Simvastatin, Rosuvastatin, Midazolam, Phenobarbital, Imatinib (Gleevac) and Verapamil to list a few. By inducing both the CYP3A4 isoform and P-glycoprotein expression, the herb decreases the concentration in blood of drugs such as Cyclosporine, Methadone, and SN-38 (CPT-11), an active metabolite of the chemotherapeutic drug Irinotecan. The concentration in blood of digoxin is also decreased due to the expression of P-glycoprotein by the herb. By inducing the CYP2C19 isoform, St. John’s wort causes a decrease in the blood concentrations of Omeprazole, a proton-pump inhibitor and Vericonazole, a fungicide [1]. By far the most glaring example of the effect of St. John’s wort is in its interference in the monitoring of warfarin therapy. Warfarin, which exists in two enantiomeric forms R-and S-warfarin, the latter being 2 to 5 times more potent than the R-isomer, is inactivated when the R-isomer is metabolized by CYP3A4 and CYP1A2 isoforms. The S-isomer is metabolized by the hepatic microsomal CYP2C9 isoform. As a consequence of this effect by St. John’s wort patients who are on warfarin therapy have a reduction in their INR (International Normalized ratio) calculated by the measurement of the prothrombin time (PT). In such patients the dose of warfarin needs to be adjusted upward in order to obtain the desirable therapeutic effect [1,14].
Ginseng, which we noted earlier a shaving salutary effects also has its share of interferences with the effects of drugs. An example can be cited of a 47-year-old patient with a mechanical heart valve who was stabilized by warfarin therapy, but then experienced a decrease in INR from 3.1 two weeks prior to starting to consume ginseng to 1.5. Two weeks after discontinuing ginseng, the INR increased to 3.3 [15]. Ginseng also causes an increase in the blood concentration of digoxin and Nifedipine, a calcium channel blocker. It also causes a decrease in the blood alcohol concentration by inducing the enzymes alcohol and aldehyde dehydrogenases [1].
In addition to St. John’s wort and ginseng several other herbs interfere with the anticoagulant effect of warfarin. Among these are the Chinese herbs (Danshen, Quilinggao, Dong quai, Go-qi-zi also known as Lycium barbarum), Angelica’s root, cloves, Devil’s claw, chamomile tea and Royal jelly, all of which cause an increase in INR [1,4]. A case report has described a 10-fold increase in INR accompanied by bleeding in a 76-yearold woman receiving long-term phenprocoumon therapy whose INR was previously in the therapeutic range (2.0-3.0). Several weeks before the bleeding episode and the increase in INR the woman began regularly consuming dried ginger and tea prepared from ginger powder. After she discontinued consuming ginger and the subsequent administration of vitamin K for over a week her INR returned to the therapeutic range for phenprocoumon therapy [16].
Gingko biloba is an herb that is widely used to improve cognitive function especially in the elderly with mild to moderate memory loss. The herb has multiple constituents among which are flavonoids which have antioxidant properties. Ginkoglide B, one of the terpene lactones found in a gingko biloba herbal mixture inhibits platelet-activating factor (PAF) and has anti-inflammatory properties. Gingko biloba,by its ability to induce CYP2C19 isoform in the liver, decreases the plasma concentration of the proton pump inhibitor Omeprazole [17]. By its ability to induce CYP2C9 isoform in the liver it also causes a statistically significant decrease in the plasma concentration of tolbutamide, a hypoglycemic agent. The herb, by inhibiting CYP3A4 isoform activity in the liver and intestine, can affect a statistically significant increase in the plasma concentration of midazolam [18].
Everyday beverages and food can interact with drugs and affect both laboratory results and therapy.
Even an apparently innocuous and widely used breakfast drink such as grapefruit juice by inhibiting the intestinal CYP3A4 isoform cancause an increase in the plasma concentrations of Felodipine, Methadone, Halofrantine, Triazolam, Ripaglinide, cyclosporine, Amiodarone, Simvastatin, Atorvastatin and other statins. By inhibiting the uptake of a drug by the intestinal organic anion-transporting polypeptide (OATAP1A2), grapefruit juice causes a decrease in the plasma concentration of Fexofenadine and Celiprolol [1,19].
The juice of Pomelo, a citrate fruit (Citrus grandis) closely related to grapefruit has similar quantities of furanocoumarins such as 6’7’-dihydroxybergamottin. The furanocoumarins by inhibiting either the Cytochrome P450 (CYP3A4) or P-glycoprotein (P-gp) activity, as could be anticipated, increases the concentration of cyclosporine and tacrolimus in blood [20,21].
Soy protein has cholesterol lowering and antioxidant properties and estrogen-like effects on the blood vessels, which is primarily attributed to the presence of isoflavones such as genistein, daidzein and glycetin [22]. It also contains saponins, which may be responsible for the increase in bile acid excretion and subsequent lowering of plasma cholesterol concentration. A meta-analysis of 38 controlled clinical studies demonstrated that soy protein caused a statistically significant decrease in the plasma concentrations of total cholesterol, low-density lipoproteincholesterol (LDL-C), triglycerides and a non-significant increase in highdensity lipoprotein-cholesterol (HDL-C) (23).A case report documented a decrease in the effect of warfarin as demonstrated by a reduced INR four weeks after the commencement of soy protein intake. After seven days the cessation of soy protein intake the INR rose to the therapeutic range for warfarin therapy [24] (Table 3).

Table 2: Lists some examples of Herbs that have toxic effects
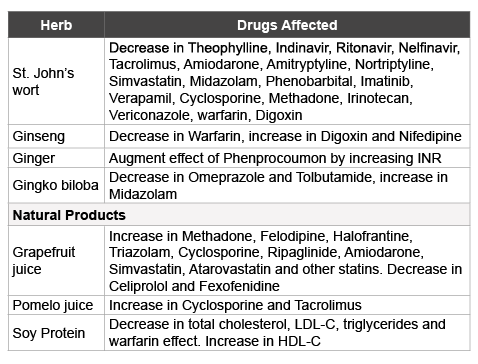
Table 3: Lists interactions of selected herbs/natural products with drugs in blood
While many herbs have salutary effects it must be recognized that some herbs are toxic. Herbs may also be contaminated with drugs and heavy metals. Strict quality control of ingredients that are present in herbal preparations as part of Good Manufacturing Practice (GMP) and testing for microbial load prior to sale ought to ensure the required quality of herbal medicines. By far, the most pressing issue facing the clinical laboratory is to recognize the effect of herbs to alter some laboratory tests and thus affect the treatment of the patient. Just as there is a compendium of drug interferences, the laboratory will be well served to maintain a listing of, at least, the most commonly used herbs and their effects on laboratory tests.
Download Provisional PDF Here
Article Type: Review Article
Citation: Narayanan S, Young DS (2016) A Perspective on Herbs and Natural Products: Impact on Therapy and Clinical Laboratory Results. J Drug Res Dev 2(3): doi http://dx.doi.org/10.16966/2470-1009.118
Copyright: © Narayanan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access