Table 1: Brine shrimp lethality bioassay of C. infortunatum extracts

Talukdar Muhammad Waliullah1†* Akter Mst Yeasmin1† Md Ashraful Alam2 Md Wahedul Islam3 Parvez Hassan3
1Molecular Cellular Biology Laboratory, Bioscience Department, GSST, Shizuoka University, 836-Oya, Suruga-ku, Shizuoka, Japan*Corresponding author: Pragati Talukdar Muhammad Waliullah, Molecular Cellular Biology Laboratory, Bioscience Department, GSST, Shizuoka University, 836-Oya, Suruga-ku, Shizuoka 422-8529, Japan, Tel: +81-080-9486-2020; E-mail: olymwt@live.com
To learn a scientific knowledge, this study was examined for evaluating anticancer, anti-microbial and pharmacological activities of natural products and the estimation of cytotoxic potency using root, leaf and stem of ethanol and ethyl acetate extracts of Clerodendrumin fortunatum (Verbenaceae) due to randomly use in customary and traditional medicine to cure common ailments such as intestinal disorder, diarrhea, tuberculosis and respiratory problems etc. The in vitro application was carried out by the bench top bioassay method using Brine shrimp lethality bioassay. The crude extracts were found to be lethal and showed significantly effective specifically the LC50 value of root of ethanol fraction was 20.845 ppm compare to the standard drug tetracycline was 14.675 ppm to brine shrimp nauplii indicating that the extracts are biologically active. The cytotoxic study of median lethal concentration (LC50) value showed that a good correlation with the antibiotic tetracycline. The comparative correlation error bars and percentage presenting that root extract of ethanol fraction was very much effective as well as the leaf extract of ethanol and stem extract of ethyl acetate fractions were also materialized significantly. This study serves as a potential template for the implications of these results for bioactivity and drug discovery.
Ethnomedicine; Median lethal concentration; Cytotoxicity; Artemiasalina; Standard deviation
The use of indigenous knowledge of traditional medical practitioners as leads provides a useful route employed in the search for novel drugs [1]. The antimicrobial potential of different medicinal plants is being extensively studied all over the world, but only a few studies have been carried out in a systematic manner [2]. Medical knowledge derived from traditional societies has motivated searches for new bioactive molecules derived from plants that show potent activity against bacterial pathogens [3]. Nowadays, some researchers are providing laboratory trials to justify for the use of the plants in folk medicine to treat various infectious diseases [4].
In the present study, C. infortunatum L. (Verbenaceae) is selected to assess its ethnobotanical importance due to randomly use as traditional medicine to cure common ailments such as intestinal disorder, diarrhea, tuberculosis and respiratory problems etc. It is reported that Bangladesh has over 5,000 medicinal plants and uses of these plants for medicinal purposes are remarkable [5]. C. infortunatumis generally used in folk, Hill tracts and rural slum dwellers as indigenous medicine, food supplement, decoration of home appliances and firewood [6,7]. Clerodendrum is a very large and diverse genus and till now five hundred and eighty species of the genus have been identified and are widely distributed in Asia, Australia, Africa and America [8]. Roots and leaf extracts of C. indicum, C. phlomidis, C. serratum, C. trichotomum, C. chinense and C. petasites have been used for the treatment of rheumatism, asthma and other inflammatory diseases [9]. It was also reported that tribals use C. inerme as an antidote of poisoning from fish, crabs and toads [10]. Roots, leaves and fresh juice of leaves of C. infortunatum were used in eliminating ascarids and tumors and also as a laxative [11]. Another study on C. chinense and C. splendens have been reported that chloroformic extracts of flower and stem were active against Plasmodium falciparum and Trypanosoma cruzi with suggesting that leaf extracts showed promising activities against T. cruzi [12].
To explore a scientific idea, this study was designed to in vitro evaluation of the cytotoxic potency carrying on, Artemia salina, brine shrimp lethality bioassay for identification and determination of chemical structure of the bioactive compounds.
The plant specimen was collected from Rajshahi University campus, Bangladesh. Identification of voucher specimen was confirmed at the taxonomical section, Department of Botany, University of Rajshahi, Bangladesh.
Root, leaf and stem were dried in shade and stored in cotton bags and then finely powdered (100 gm) separately with the help of a grinder. Each ground material was soaked in 500 ml ethyl alcohol and ethyl acetate separately for 24-72 h and filtered (Whatman no 1). The filtered was then allowed to vaporize in rotary evaporator until completely dried and kept in a refrigerator. Then (100 mg and 50 mg) dried extract, for further study, was weighed and dissolved in 10 ml of respected solvents for dilution. The concentration of the final extract was 100 µg/10 µl and 50 µg/10 µl.
Brine shrimp lethality bioassay is a bench top bioassay method for evaluating anticancer, anti-microbial and pharmacological activities of natural products [13,14]. Here in vivo lethality of a simple zoological organism (brine shrimp nauplii) is used as a convenient monitor for screening of a fractionation in the discovery of new bioactive natural products [15,16]. Generally, the median effective dose (ED50←) values for cytotoxicity are one tenth (1/10) of median lethal dose (LD50) values in the brine shrimp testn [17]. Thus, it is possible to detect and then monitor the fractionation of cytotoxic, as well as 3PS (P388) (in vivo murine leukemia) active extracts using the brine shrimp lethality bioassay [18]. Bioactive compounds are almost always toxic in high doses. According to the American National Cancer Institute (NCI), a crude plant extract is considered to be cytotoxic if its CC50 value on mammalian cells is <30 µg/mL [19]. On the other hand the cut-off point to consider a crude plant extract non-toxic in the brine shrimp toxicity assay is LC50>100 µg/mL [20,21]. There is a positive correlation between toxicity against brine shrimp and cytotoxicity against 9KB (human nasopharyngeal carcinoma) (P=0.036 and kappa=0.56), as well as to the median lethal concentration (LC50) of the acute oral toxicity assay in mouse (r=0.85; P<0.05) [22,23].
Small amount of brine shrimp eggs were hatched at simulated sea water. About 38 gm pure NaCl (also known as sea salt) was dissolved in 1 L of distilled water to make simulated sea water. The pH should have at 8.3-8.7. Then a little number of collected shrimp eggs was liberated into water and continuous ventilation for oxygen supply was attached to the tank of hatching. A continuous lighting system was also assured.
Ethyl alcohol and chloroform fraction of C. infortunatum root, leaf and stem extracts were applied against brine shrimp nauplii. Initially, 2 mg extracts of each part separately were dissolved in 100 µl of pure dimethyl sulfoxide (DMSO) to make hydrophilic before adding 19.98 ml of water to get a concentration for each of 200 ppm which was used as stock solution-A. From the stock solution A, 10 ml was taken (that was with a concentration of 200 ppm) and diluted up to 20 ml with brine water to obtain a concentration of 100 ppm and this is indicated a stock solution-B and from the stock solution B, 10 ml was taken (that was with a concentration of 100 ppm) and diluted up to 20 ml with brine water to obtain a concentration of 50 ppm for making stock solution-C. This is called the serial dilution method. Then the following concentrations were made 200, 100 and 50 ppm. During experiments according to the dosemortality effects dose ranges were made different for some conveniences in achieving a good result since the extracts were active against the brine shrimp nauplii.
For each concentration, one test tube containing the same volume of DMSO diluted up to 10 ml with sea-water and 30 shrimp nauplii was used as negative control group. It was used to verify the validity of the test. When the nauplii in the control showed a rapid mortality, then the test is considered to be invalid as the nauplii might die due to reasons other than the cytotoxicity of the compounds.
In each of the five test tubes, 10 ml brine shrimp solution (3.8%) was taken, containing 30 brine shrimp nauplii with the help of a micropipette, specific volumes of each sample were transferred from the stock solution-B to the respective vials to get, final concentrations of 200, 100 and 50 µg/ ml. The volumes of DMSO in these test tubes should not exceed 10 µl/ ml of the brine solution, because above this concentration toxicity due to DMSO may arise.
After 24 hours, the test tubes were observed. The number of survived nauplii in each test tube was counted and the results were noted. From this, percentage of mortality of brine shrimp nauplii was calculated at each concentration for each sample and the results are presented in the.
The dose mortality data were analyzed statistically by Probit analysis [24].The effectiveness or the dose mortality relationship (concentrationmortality relationship) of plant product is usually expressed as a median lethal concentration (LC50) value. This represents the concentration of the chemical that produces death in half of the test subjects after a certain exposure period.
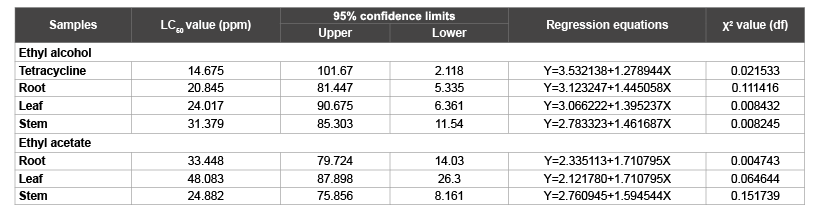
The results of the brine shrimp lethality bioassay are presented. The ethyl alcohol and ethyl acetate extracts of root, leaf and stem of C. infortunatum were subjected to apply against the brine shrimp (A. salina) nauplii for the detection of their biological activity. Several doses were selected by a pilot (Ad Hoc) experiment and a final experiment was set up with 3 replications and control doses. From the experiment, it was revealed that each of the test samples showed different mortality rates at different concentrations. The mortality rates of brine shrimps nauplii increased with the raise of the concentration of the tested crude extracts. The LD50 values along with 95% confidence limits, regression equations and chi-squared value of the crude extracts (root, leaf and stem) and the tetracycline for 24 h of exposure were determined, using Probit analysis. The results have been tabulated in the Table 1. Regression lines drawn by plotting log concentrations of the extracts of C. infortunatum against probit mortality reveal linear correlation between log doses and empirical probit mortality (Figures 1-3).
The LC50 value of root, leaf and stem were 20.845, 24.017 and 31.379 ppm for ethyl alcohol, 33.448, 48.083 and 24.882 ppm for ethyl acetate respectively and 14.675 ppm for tetracycline (Table 1).
The 95% confidence limits of root, leaf and stem were 5.335 to 81.447, 6.361 to 90.675 and 11.543 to 85.303 for ethyl alcohol, 14.017 to 79.033, 26.302 to 87.898 and 8.161 to 75.856 for ethyl acetate respectively and 2.118 to 101.678 for tetracycline (Figure 1).
The regression equation of root, leaf and stem were Y=3.123247+1.445058X, Y=3.066222+1.395237X and Y=2.783323+1.461687X for ethyl alcohol (Figures 2A- 2C), Y=2.335113+1.710795X, Y=2.121780+1.710795X and Y=2.760945+1.594544X for ethyl acetate respectively (Figures 3A-3C) and while Y= 3.532138+1.278944X for tetracycline (Figure 1).
The χ2 values (df) of root, leaf and stem 0.111416, 0.008432 and 0.008245 for ethyl alcohol, 0.004743, 0.064644 and 0.151739 for ethyl acetate respectively and 0.021533 for tetracycline with 1 degrees of freedom (Table 1). The median lethal concentration (LC50) of cytotoxic bioassay test was compared to the standard antibiotic tetracycline with correlation error bars and percentage (Figure 4).
All of the crude extracts were found to be lethal and showed significantly effective to brine shrimp nauplii indicating that the extracts are biologically active.
From the present study the mortality rates of brine shrimps nauplii increased with the raise of the concentration of the tested crude extracts. The LC50 values of the crude extracts of root, leaf and stem were found to be 20.845, 24.017 and 31.379 ppm for ethyl alcohol; 33.448, 48.083 and 24.882 ppm for ethyl acetate respectively and 14.675 ppm for the tetracycline. 95% confidence limits, regression equation and χ2 values were also significant. It is the general agreement [25,26] who studied the lethality test of Euphorbia hirta. Recorded cytotoxicity observation to brine shrimp nauplii also supports our previous antimicrobial activities of C. inforunatum [27-29] and C. viscosum [30]. Consecutive chain of study it is furthermore represents our earlier results of insecticidal, repellent and mortality activity of C. inforunatum [31,32].

Table 1: Brine shrimp lethality bioassay of C. infortunatum extracts
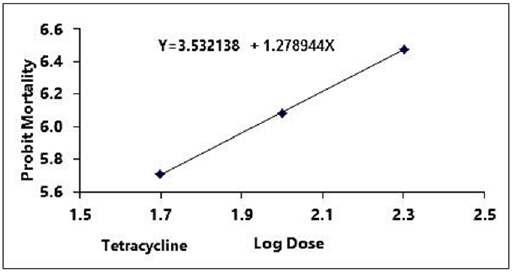
Figure 1: Probit regression lines between probit mortality of brine shrimps nauplii and log dose (+1 mgcm-2) of tetracycline after 24 h exposure
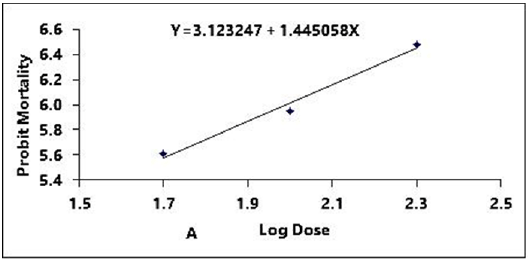
Figure 2A: Probit regression lines between probit mortality of brine shrimps nauplii and log dose (+1 mgcm-2) of root of ethyl alcohol extracts after 24 h exposure
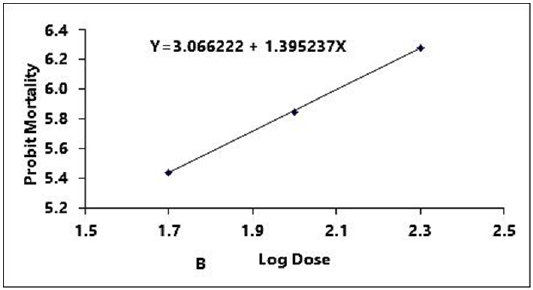
Figure 2B: Probit regression lines between probit mortality of brine shrimps nauplii and log dose (+1 mgcm-2) of leaf of ethyl alcohol extracts after 24 h exposure
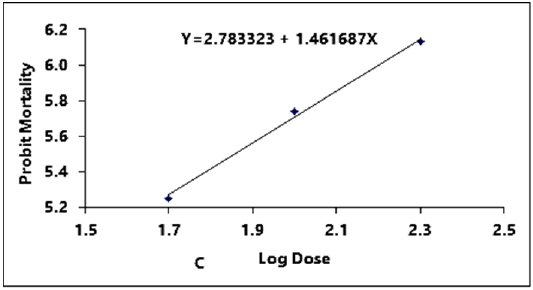
Figure 2C: Probit regression lines between probit mortality of brine shrimps nauplii and log dose (+1 mgcm-2) of stem of ethyl alcohol extracts after 24 h exposure
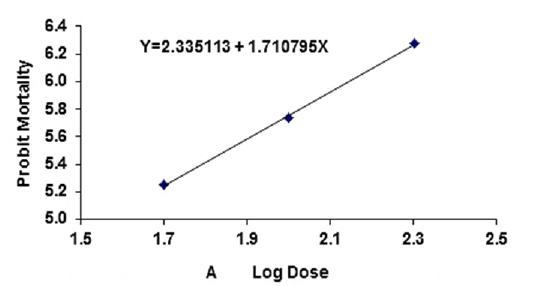
Figure 3A: Probit regression lines between probit mortality of brine shrimps nauplii and log dose (+1 mgcm-2) of A root of ethyl acetate extracts after 24 h exposure
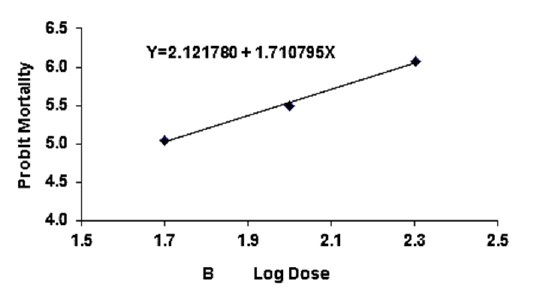
Figure 3B: Probit regression lines between probit mortality of brine shrimps and log dose (+1 mgcm-2) of B leaf of ethyl acetate extracts after 24 h exposure
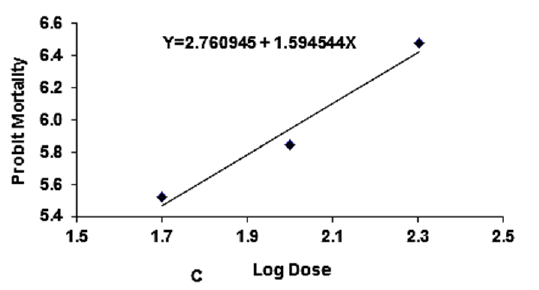
Figure 3C: Probit regression lines between probit mortality of brine shrimps nauplii and log dose (+1 mgcm-2) of C stem of ethyl acetate extracts after 24 h exposure
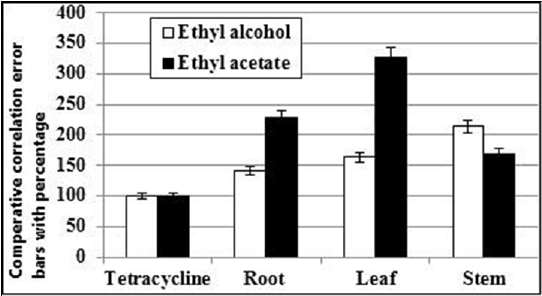
Figure 4: The comparative median lethal concentration (LC50) with correlation error bars and percentage
The cytotoxic study of median lethal concentration (LC50) value showed that a good correlation with the antibiotic tetracycline. Recently we demonstrate the cytotoxic correlation among ethyl alcohol and chloroform fraction of C. infortunatum with the antibiotic tetracycline [33]. From the comparative correlation error bars and percentage, we understood that root extract of ethyl alcohol fraction was very much effective. Of course leaf extract of ethyl alcohol and stem extract of ethyl acetate fractions were also materialized significantly. Cytotoxic evidence of biologically active nature of the extracts of C. infortunatum was emerged from the result of the brine shrimp lethality bioassay. However, the potential chemical components of this plant showing bactericidal, fungicidal or other toxicological activities are yet to be identified.
The above findings specify that C. infortunatum possess promising biological properties and appears to be an effective material for the development of antimicrobial drugs and eco friendly bio-pesticides. Thus, this study serves as basis for further research to lead compounds to be isolated so that may be as a template for the implications of these results for bioactivity and drug discovery potential of herbal products are discussed.
Institute of Biological Sciences, University of Rajshahi has provided the lab facility and supported by Ministry of Science and Information & Communication Technology, Government of the Peoples Republic of Bangladesh under the project of Research and Development (R&D), Grant no. Bitojopromo/sha-12/goupro-8/2008/268.
We declare that we have no conflict of interest.
Download Provisional PDF Here
Article Type: Research Article
Citation: Waliullah TM, Yeasmin MA, Alam MA, Islam MW, Hassan P (2016) Analysis of Toxicity Assay of Crude Drug; Clerodendrum infortunatum L. J Drug Res Dev 2(2): doi http://dx.doi.org/10.16966/2470-1009.116
Copyright:© 2016 Waliullah TM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access