Introduction
Liver is an organ having both secretary and excretory functions.
Most of synthetic and metabolic functions of liver are performed by
hepatocytes. Hepatocytes play important role in the metabolism and
storage of proteins, carbohydrates, fats, vitamins and hormones. Plasma
proteins are also synthesized by liver parenchymal cells. Liver can both
synthesize and remove cholesterol from body. Non-essential amino acids,
when required, are also synthesized by liver [1,2].
Liver enzymes included SGOT (Serum glutamic oxaloacetic
transaminase), SGPT (Serum glutamic pyruvic transaminase), ALP
(Alkaline phosphatase) and LDH (Lactate dehydrogenase) are present
within the cells of liver. These enzymes are released in circulation when
hepatocytes damage or die. Plasma half-life of SGOT and SGPT is 16-18
hrs and 42-48 hrs respectively while the half-life of mitochondrial SGOT
is 87 hrs. These enzymes act as marker of liver injury. Bilirubin is formed
by enzymatic breakdown of haemoglobin in reticuloendothelial system.
The plasma level of bilirubin also raised in case of liver impairment [3].
Paracetamol (N-acetyl p-aminophenol, 4-hydroxy acetanilide), also
called acetaminophen, is commonly used analgesic and antipyretic drug.
At therapeutic dose, it is the most safe and effective drug. When high dose
of drug is taken accidentally or for suicidal attempt, then this toxic dose
of paracetamol causes centrilobular necrosis in liver causing liver failure
and finally death occur [4,5]. Oxidative stress, nitrotyrosine formation,
inflammatory cytokines and mitochondrial permeability transition
considered to be involved in paracetamol induced hepatotoxicity.
Paracetamol is basically metabolized through conjugation with glucuronic
acid and sulphate, but a minor pathway is also through Cyp4502E1 enzyme
system which converts paracetamol to toxic metabolite N-acetyl -p
-benzoquinone imine (NAPQI). NAPQI is detoxified through glutathione
(GSH) pathway by the formation of glutathione conjugate. In case of over
dose of paracetamol, high concentration of NAPQI is produced, which
causes depletion of GSH stores. So this toxic metabolite reacts with –SH
group of normal cell proteins and form paracetamol protein adducts and
causing cell death [6,7].
Amino acids are having numerous protective effects which can
help body to combat stress condition. They are abundantly present
in protein rich diet such as poultry, eggs, wheat, broccoli, garlic,
onions, red pepper, soyabean and nuts. N-acetylcysteine, a precursor
of glutathione, is used to prevent paracetamol induced hepatotoxicity
[8]. Glutathione is made up of three amino acids; glycine, glutamic
acid and cysteine. L-Cysteine is a non-essential sulphur containing
amino acid which can be synthesized in human body from amino acid
methionine.In case of oxidative stress due to protein malnutrition
or hepatic depletion of glutathione, cysteine restores gamma
glutamyl cysteine ligase enzyme [9]. Cysteine, being a precursor
of glutathione (GSH), possesses powerful antioxidant properties
and acts as an extracellular reducing agent [10]. Lysine is also an
essential amino acid and useful in heavy metals chelation, activation
of protease enzymes, production of hormones and antibodies which
strengthen the immune system [11]. Glycine is a non-essential
amino acid because in human body it can be produced by liver from
other amino acids. Glycine provides defense to body against variety
of conditions such as sepsis, hemorrhage and attack of endotoxins.
Glycine also protects the liver, kidney, skeletal muscles and heart
against reperfusion injury due to ischemia, cold conditions various
toxic compounds and drugs [12].
The main objective of this study was to determine the efficacy of various
amino acids in paracetamol induced hepatotoxic animals. Cysteine, Lysine
and Glycine were evaluated for their relative effectiveness in ameliorating
hepatic damage caused by paracetamol, the most commonly prescribed
analgesic and anti-pyretic drug.
Materials
Chemicals
Three types of amino acids, L-cysteine (Daejung), lysine (Fluka Granite)
and glycine (Merck Pharmaceuticals) were used for this experimental
study to check their hepatoprotective effect while paracetamol was used
to induce hepatotoxicity. All solvents used are of analytical grade. Distilled
water was obtained from the distillation plan in our laboratory.
Experimental animals
Albino rabbits weighing 1 to 2 kg were purchased from the local market.
The animals were kept in the animal house of the Faculty of Pharmacy,
University of the Punjab, Lahore. They were acclimatized for a period of
one week before starting the experiment. The rabbit were fed fresh green
fodder and water ad libitum.
Collection of blood
The rabbits were placed in a wooden box and the ear of rabbit was
carefully shaved. The marginal vein of the ear was made prominent by
rubbing the ear. A fine cut was made with sharp edged surgical blade and
the blood was collected in centrifuge tube. The collected blood was kept in
ice cold water for 10 minutes and was centrifuged for 15 minutes at 4000
revolutions per minute. Finally the serum was collected with the help of
micro pipette in Eppendorf and labeled.
Experimental design
The rabbits were mainly divided into two groups; acute treated group
and chronic treated group. Each group was further divided into 5 subgroups
and in each sub-group 6 rabbits (n=6) were included for each
experimental study. Before treatment, all rabbits were investigated both
physically and biochemically and only healthy rabbits were selected for
study.
Acute Treated Group (ATG GROUP)
In each treatment sub-group, 6hrs after giving respective protocol,
blood samples were drawn and changes in liver function test (LFT)
parameters i.e. SGPT, SGOT, ALP, LDH, and total bilirubin (TB) were
observed.
Group I (Control Group): This group was given distilled water
through intragastric tubing.
Group II (Toxic Group): Paracetamol suspension prepared by
dissolving 3g/kg paracetamol powder in water using tragacanth as
suspending agent was administered to rabbits through intragastric tubing
to induce acute hepatotoxicity.
Group III (L-cysteine Treated Group): L-cysteine (600mg/kg) was
injected intraperitoneally10minutes before giving acute paracetamol toxic
dose.
Group IV (Lysine Treated Group): L-lysine (10mg/kg) was injected
intraperitoneally10 minutes before giving acute paracetamol toxic dose.
Group V (Glycine Treated Group): Glycine (800mg/kg) was
administered orally through intragastric tubing10minutes before giving
acute toxic paracetamol dose.
Chronic treated group
In this study group, each sub-group was treated with a course of thirty
days of its respective protocol and then blood samples were drawn and
changes in liver function test (LFT) parameters (ALT, AST, ALP, LDH,
and total bilirubin) were observed.
Group I (Control Group): This group was given placebo (water) orally
through intragastric tubing for 30 days.
Group II (Toxic Group): Paracetamol suspension, prepared by
dissolving 75mg/kg/d paracetamol powder in water using tragacanth as
suspending agent, was administered to rabbits through intragastric tubing
for 30 days in animals with normal liver function tests.
Group III (L-cysteine Treated Group): L-cysteine (200mg/kg) was
injected intraperitoneally to paracetamol treated rabbits during the last
week of their 30 days toxicity protocol.
Group IV (Lysine Treated Group): L-lysine (3mg/kg) was injected
intraperitoneally to paracetamol treated rabbits during the last week of
their 30 days toxicity protocol.
Group V (Glycine Treated Group): Glycine (266.6mg/kg) was
administered orally through intragastric tubing to paracetamol treated
rabbits during the last week of their 30 days toxicity protocol.
Statistical analysis
Data was analyzed by using SPSS version 21.0. All the liver profile
parameters were noted as standard deviation of the mean. Different
experimental groups were compared by applying ANOVA post-hoc
tukey’s test using statistical tool SPSS®
version 21. A statistical value of p ≤
0.05 was considered significant.
Results
Effects of paracetamol, L-Cysteine, Lysine and Glycine on
hepatic profile in acute treated study
In acute toxicity study, toxic effects of paracetamol and protective
effects of three amino acids including L-Cysteine, Lysine and Glycine were
observed on SGOT, SGPT, ALP, LDH and total bilirubin levels (Table 1,
Figure 1A). After applying statistical tools between toxic group(Group
II) and control group (Group-1), a highly significant increase (p<0.05)
in the levels of SGOT(84% ↑), SGPT (70% ↑), ALP(22% ↑), LDH (80% ↑)
and total bilirubin (57% ↑) were observed in toxic Group. When Cysteine
treated group (Group III) was compared with Toxicgroup (Group II),
results showed highly significant decrease (p<0.05) in plasma level of
SGOT (60% ↓), SGPT(41% ↓), ALP (63% ↓), LDH (73% ↓) and total
bilirubin (59% ↓). Similarly Lysine treated group (Group IV) showed a
significant decrease (p<0.05) in the plasma levels of SGOT (30% ↓), SGPT
(61% ↓), ALP (49% ↓), LDH (73% ↓) and on total bilirubin (57% ↓) as
compared to toxic group (Group II), but glycine treated group (Group V)
did not show any significant (p>0.05) change in SGOT (18% ↓) level while
significant decrease (p<0.05) in the level of SGPT (33% ↓), ALP (26% ↓),
LDH (66% ↓) and total bilirubin (46% ↓).
Effects of paracetamol, L-Cysteine, Lysine And Glycine on hepatic
profile in chronic toxicity study
In case ofchronic toxicity study, toxic effects of paracetamol and
protective effects of three amino acids including L-Cysteine, Lysine and
Glycine were observed on SGOT, SGPT, ALP, LDH and total bilirubin level
(Table 2, Figure 1B). After applying statistical tools between toxic control
group (Group II) and Control group (Group-1), significant increase
(p<0.05) in the levels of SGOT (64% ↑), SGPT (103% ↑), ALP (15% ↑),
LDH (23% ↑) and total bilirubin (33% ↑) were observed. Comparison of
Cysteine treated group (Group III) compared with Control group (Group
II) showed significant decrease (p<0.05) in plasma level of SGOT (25%
↓), SGPT(29% ↓), ALP (19% ↓), LDH (28% ↓) and total bilirubin (20% ↓).
Similarly Lysine treated group (Group IV) showed a significant decrease
(p<0.05)in the plasma levels of SGOT (21% ↓), SGPT (25% ↓), ALP (12% ↓)
and non significant change in the level of LDH (23% ↓) and total bilirubin
(17% ↓) as compared to positive control group (Group II). Glycine treated
group (Group V) did not show any significant (p>0.05) change in SGOT
(18% ↓) level,LDH (19% ↓) and total bilirubin (16% ↓) while significant
decrease (p<0.05) in the level of SGPT (25% ↓), ALP (15% ↓).
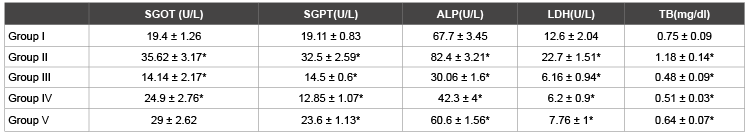
Table 1: Effects of l-cysteine, lysine, glycine on liver profile in paracetamol treated rabbits in acute treated study
1. Group I: Control Group, Group II: Toxic Group,Group III: Cysteine treated group, Group IV: Lysine treated group, Group V: Glycine treated Group
2. Values are represented as Mean ± SEM
3. * represents P<0.05
4. Group I is compared with Group II. Group II is compared with Group III, IV and V
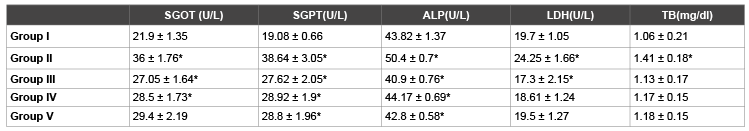
Table 2: Effects of l-cysteine, lysine, glycine on sgot level in paracetamol treated rabbits in chronic toxicity study
1. Group I: Control Group, Group II: Toxic Group, Group III: Cysteine treated group, Group IV: Lysine treated group, Group V: Glycine treated Group
2. Values are represented as Mean ± SEM
3. * represents P<0.05
4. Group I is compared with Group II. Group II is compared with Group III, IV and V
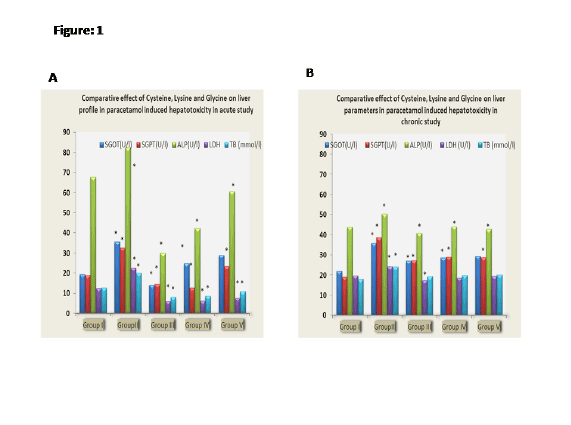
Figure 1A: Comparative effect of Cysteine, Lysine and Glycine on liver
profile in paracetamol induced hepatoxicity in acute study
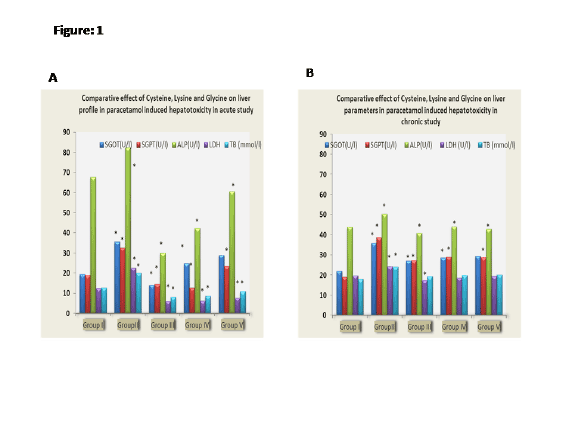
Figure 1B: Comparative effect of Cysteine, Lysine and Glycine on liver
parameters in paracetamol hepatotoxicity in chronic study.
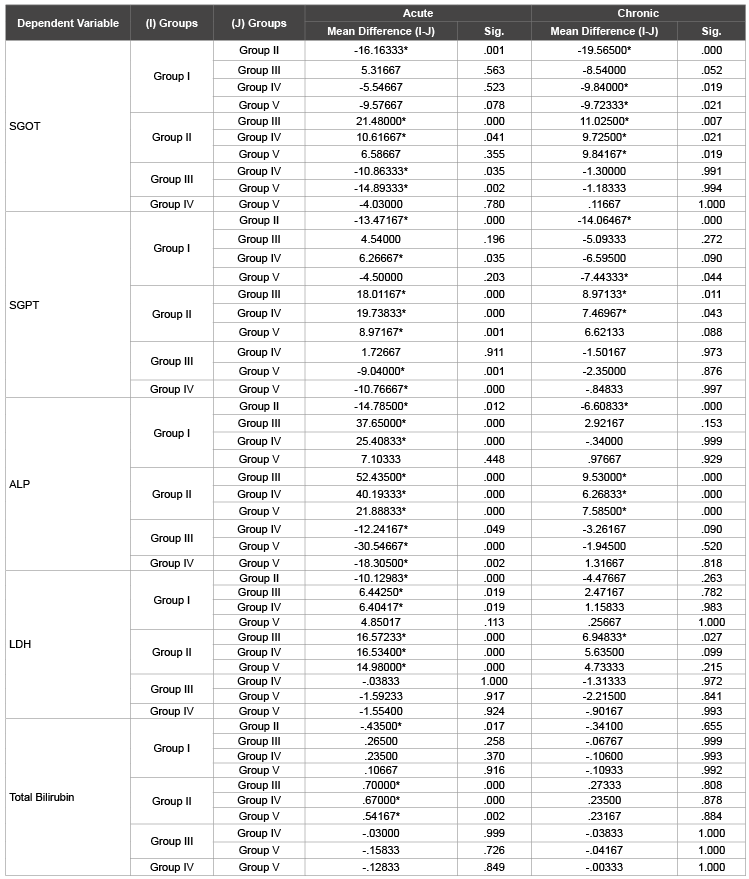
Table 3: Multiple comparision of treated groups in acute and chronic study
1. Group I: Control Group, Group II: Toxic Group, Group III: Cysteine treated group, Group IV: Lysine treated group, Group V: Glycine treated Group
* represents P<0.05
Discussion
Drug induced liver injury (DILI) is a major limiting factor in use of
drugs for therapeutic purpose. It is the most common disease condition
of the liver [13]. The hepatotoxins have a unique pathway though which
they damage liver cells. Individual factors also play a key role to make a
patient susceptible for drug induced liver injury (DILI). By knowing these
factors through advancement of toxicogenomics and proteomics, damage
can be minimized [14]. A number of agents have already been marketed
to prevent drug induced hepatotoxicity. Among them, amino acids
play a central role in preventing cell damage. In this study, comparative
effectiveness of cysteine, lysine and glycine to combat hepatotoxins was
observed.
In Table 3, comparative study suggests that both cysteine and lysine
are equally effective depending upon the pathways suggested below but
glycine was not equally effective in reducing SGOT levels, however glycine
plays an important role in hepatic inflammatory pathological conditions.
In cysteine treated group (Group III), highly significant (P<0.05) decrease
in the levels of SGPT, SGOT, ALP, LDH and bilirubin was observed in
both acute and chronic toxicity study. Cysteine is required for synthesis of
glutathione and PAPS (3-phosphoadenosine-5-phosphosulphate). PAPS
is required for sulfation of paracetamol while glutathione is required
for detoxification of NAPQI. So cysteine prevents paracetamol induced
hepatotoxicity by shifting paracetamol metabolism towards safer side
[15].Glutathione possess antioxidant property by reducing activity of
reactive oxygen species. Cysteine also recovers the body from oxidative
stress by increasing level of gamma glutamyl cysteine ligase enzyme which
is required for glutathione synthesis [9]. Lysine treated group (group-IV)
also showed a similar significant reduction in levels of SGOT and LDH,
very significantly reduction in the level of SGPT while a highly significant
reduction in ALP level. L-N6
-(1-iminoethyl)-lysine (L-NIL) is a lysine
derivative. It has also been suggested that L-NIL acts on active site of
iNOS enzyme and suppresses its activity. L-NIL prevents iNOS mRNA
expression by inactivating NF-B, which regulates iNOS mRNA expression.
The L-NIL also maintained GSH levels by reducing NO production in the
liver because reactive nitrogen species are scavenged by GSH [16]. It also
restores the perfusion of sinusoids. L-NIL also prevents the hepatic insult
by reducing the bioactivation of paracetamol [17].
Treatment with glycine (Group V) showed a little different result
as shown in (Table 3) and (Figure 1). In both acute and chronic study,
only the level of SGPT was significantly reduced. Glysine being a part of
Glutathione (GSH), a tripeptide of glutamic acid, cysteine and glycine,
protects cells from free radical associated oxidative stress [18]. Glycine
also improves survival in rat liver transplantation [19]. Glycine prevents
phagocytosis, hepatocellular injury, fibrosis and apoptosis caused
by intoxication with hepato toxic drugs [20]. Glycine also possesses
antiperoxidative effects by restoring the activity of superoxide dismutase,
catalase and glutathione peroxidase thus protecting cells from oxidative
stress of NAPQI. Glycine also maintains lipid peroxidation (LPO) level
of liver [1]. This study also confirms the role of glycine in maintaining
the antioxidant activity of liver. In paracetamol induced hepatotoxicity,
kupffer cells are activated resulting in release of cytokines. Glycine gated
chloride channels are located on kupffer cells. Thus cytokines production
is inhibited as glycine inactivates kupffer cells by hyperpolarizing cellular
membrane. So glycine possesses anti-inflammatory activity and improves
hepatic pathologic conditions [21]. Glycine also increases portal blood
flow, production of bile, the hepatic microcirculation which improves
cytochrome P450 activity and significantly reduces the levels of ALT,AST
during hepatocellular injury [22].
Conclusion
It is inferred that cysteine, lysine and glycine markedly reduce the
elevated liver enzymes in paracetamol induced hepatotoxic rabbits. In
both acute and chronic toxicity study, there was significant reduction in
liver enzymes in cysteine and lysine treated groups as compared to the
glycine treated animals.
Conflict of Interest
It is declared that there is no conflict of interest.






