Figure 1: Distribution of patients according to the results of the IPS.


Ibtissem Ben Nacef Dayssem Khelif* Imen Rojbi Youssef Lakhoua Nadia Mchirgui Karima Khiari
Department of Endocrinology, University Hospital of Charles Nicolle Tunis, Tunisia*Corresponding author: Dayssem Khelifi, Department of Endocrinology, University Hospital of Charles Nicolle Tunis, Tunisia, E mail: dayssem.khelifi@gmail.com
Objective: The aims of our study are to identify the prevalence of LEOPAD in type 2 diabetes, to study the predictive factors of LEOPAD and to demonstrate the importance of establishing a specialized screening consultation in diabetic patients with LEOPAD and with high cardiovascular risk.
Research design and methods: We carried out a prospective and descriptive study. The study involved 150 patients with type 2 diabetes who were followed on a regular basis. The different epidemiological, clinical and biological data were collected patient’s medical file. Each patient was investigated for cardiovascular risk factors. A thorough physical examination was performed in addition to biological assessment (fasting plasma glucose, HbA1c, lipid profile). After consent had been obtained, all patients underwent the ankle-brachial systolic pressure index (ABI) test.
Results: The mean age of our patients was 57.46 ± 8.04 of the 150 patients, 24.7% had abnormal ABI suggestive of lower limb ischemia. Among them, 8.7% had an ABI of 1, 3, a presumed marker of vascular stiffness. The mean age of the LEOPAD population was 58 years. LEOPAD was predominantly asymptomatic in our population (88.1%). Only 5 patients (11.9%) had intermittent claudication. The comparative study of patients with LEOPAD showed a statistically conversely significant correlation between LEOPAD and lower levels of HDL. However, there is no statistically significant correlation with the following factors: smoking, sex, hypertension, dyslipidemia, obesity or overweight android pattern, the presence of microalbuminuria, renal failure and sedentary lifestyle. In terms of glycemic control, it did not show a statistically significant difference between the two groups. Moreover, it was noted that the patients with LEOPAD had had more micro vascular complications compared to patients without LEOPAD.
Conclusion: Lower extremity occlusive peripheral artery disease occurred more commonly than it was expected in the diabetic population, associated with a high cardiovascular comorbidity. The sensitivity, specificity and simplicity of the ABI measurement promote its routine use and integration into the clinical approach of high-risk LEOPAD patients, especially the diabetics.
Lower extremity occlusive peripheral artery disease (LEOPAD) is defined as partial or complete obstruction of one or more peripheral arteries of the lower limbs, generally related to atherosclerosis. It is estimated that more than 200 million individuals are affected by lower extremity artery disease worldwide. This prevalence has increased between 2000 and 2010 by 25%, especially in low/middle income countries [1]. Another recent systematic review estimated that 238 million people were living with LEOPAD in 2015: 64 million living in high-income countries and 172 million living in low- and middleincome countries [2]. It is associated with many cardiovascular risk factors and is very common in people with diabetes mostly type 2. It is 2-4 times more frequent in people with type 2 diabetes than in the general population [3,4]. The natural history of LEOPAD seems to be silent in most cases and that makes its prevalence underestimated. However, it may involve inexorable progression to amputation unless there is an intervention that results in the improvement of arterial perfusion.
Another challenge in the screening and early management of LEOPAD comes from the fact that asymptomatic LEOPAD represents an important and independent predictor of cardiovascular morbidity and mortality [5] (death from myocardial infarction, acute coronary artery disease, and cerebrovascular accidents) after being adjusted for age and other risk factors. Therefore, the early diagnosis of LEOPAD in high-risk patients makes it possible to identify those who are asymptomatic with other cardiovascular complications and thus preventing them from potential risk of increased morbidity and mortality.
Measurement of the resting ankle-brachial systolic pressure index (ABI) should be performed in high-risk patients. There is a general, but not absolute, correlation between symptoms and the site and severity of LEOPAD, with severity being estimated from the ankle brachial index. That is a reliable, reproducible, simple, and inexpensive and can be performed at the bedside [5,6].
The aims of our study are to identify the prevalence of LEOPAD in type 2 diabetes, to study the predictive factors of LEOPAD and to demonstrate the importance of establishing a specialized screening consultation in diabetic patients with LEOPAD and with high cardiovascular risk.
We carried out a prospective and descriptive study over a period of one year. The study involved 150 patients with type 2 diabetes who were followed on a regular basis. The different epidemiological, clinical and biological data were collected patient’s medical file. Each patient was investigated for cardiovascular risk factors (age, sex, smoking, dyslipidemia, obesity, menopause, and coronary artery disease inheritance), their previous medical history, and prescribed medicines as well as identifying the characteristics of diabetes (how controlled the diabetes was, treatment prescribed, and complication assessment) A thorough physical examination was performed which included weight, height, waist circumference, body mass index (BMI), cardiovascular and neurological examinations in addition to biological assessment (fasting plasma glucose, HbA1c, lipid profile). After consent had been obtained, all patients underwent the ABI test. We used a pocket doppler and a sphygmomanometer to measure ABI. The measurement of the ABI was performed on both legs. ABI of at least one of the two legs of less than 0.9 was considered to be abnormal. In addition to this screening test, a questionnaire (Edimberg) was filled, and a vascular and neurological clinical examination were carried out for each patient.
This study included type 2 diabetic patients, of both sexes, aged under 50 with at least one risk factor for atherosclerosis or aged 50 or over with or without risk factors, regardless of the duration of the diabetes.
The exclusion criteria were patients who had already been diagnosed with lower extremities arthritis, patients over 80 years old, patients who had undergone an arterial echo-doppler of lower extremities of less than 5 years and patients with coronary artery disease, renal vascular disease, or known carotid artery or previous history of stroke.
Data were entered and analyzed using SPSS software. Quantitative variables were expressed as mean ± standard deviation and qualitative values as numbers and percentages. Comparisons between quantitative variables were made using the analysis of variance test or the Student test. Comparisons between qualitative variables were made using the X² (chi-square) test.
Before starting data collection, approval from the Charles Nicolle Hospital ethics committee was obtained. Patients were invited to participate in this study. After clearly explaining the purpose of the study, participants signed a consent form.
The mean age of our patients was 57.46 ± 8.04 years ranging from 38 years to 80 years. The majority of our patients (82.5%) were older than 50 years old. There was a slight male predominance (57% versus 43%) with a sex ratio of 1, 3. The clinical and biological characteristics are summarized in table 1.
| Variables | Population (n=150) |
| Meanage (years) | 57.46 (38-80) |
| Gender (M/F) | 57%/43 (n=85/65) |
| Duration of diabetes (years) | 10.45 |
| Hypertension | 57.3% (n=86) |
| Smoking | 40% (n=60) |
| Android fat Distribution | 73.2% (n=101) |
| Obesity | 39.3% (n=59) |
| Dyslipidemia | 40.7% (n=61) |
| Sedentarity | 28.7% (n=43) |
Table 1: Characteristics of the general population.
Mean diabetes duration was 8.42 ± 8.04 years with extremes ranging from 5 to 20 years. The mean HbA1c level was 8.19 ± 1.74% with extremes ranging from 5.60 to 14.9%. The diabetes was poorly controlled in 71.9% of patients. Patients had medical treatment (oral antidiabetic agents alone) in 55.3%, insulin and oral antidiabetic agent in 26.7%, and insulin alone in 16.7%. Complication assessment revealed the presence of one or more of the micro vascular complication (Table 2).
| Microvascular complications | Population (n=150) |
| Diabetic retinopathy | 31 (20.8%) |
| Diabetic nephropathy | 28 (18.7%) |
| Peripheral diabetic neuropathy | 74 (49.3%) |
| Vegetative diabetic neuropathy | 20 (13.3%) |
Table 2: Prevalence of micro vascular complications.
The assessment of cardiovascular risk using a scoring system SCORE (Systematic Coronary Risk evaluation) revealed an average score of 1.85 ± 1.81 with extremes ranging from 0 to 10.
In 99.3% of patients, at least two risk factors cardiovascular risk have been identified. The edimbourg questionnaire revealed the presence of intermittent claudication in 18 patients (12% of the study population). On palpation of posterior pedal and tibial pulses, 42 patients (28%) had at least one weak or absent pulse.
The mean values of the ankle-brachial systolic pressure index (ABI) in the general population are illustrated in the following table 3.
| Right ABI | Left ABI | |||
| Mean ± SD | Extremes | Mean ± SD | Extremes | |
| General population (n=150) | 1.07 ± 0.15 | 0.37 - 1.52 | 1.08 ± 0.14 | 0.66 -1.87 |
| Males (n=85) | 1.08 ± 0.147 | 0.37 - 1.45 | 1.07 ± 0.153 | 0.66 -1.87 |
| Females (n=65) | 1.05 ± 0.153 | 0.46 - 1.52 | 1.08 ± 0.14 | 0.83 -1.41 |
Table 3: Mean values ABI in the general population.
ABI: ankle-brachial systolic pressure index. SD: Standard Deviation
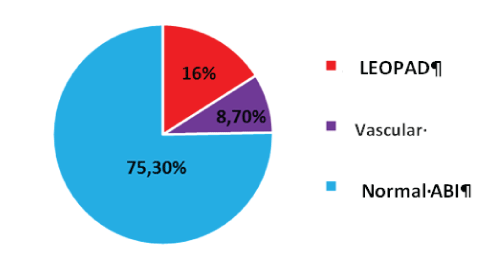
Of the 150 patients, 24.7% had abnormal ABI suggestive of lower limb ischemia. Among them, 8.7% had an ABI of 1, 3, a presumed marker of vascular stiffness.
The result of the LEOPAD screening with ABI is represented by the following figure 1.

Figure 1: Distribution of patients according to the results of the IPS.
The mean age of the LEOPAD population was 58 years, the sex ratio was 0.88. The mean duration of diabetes onset in LEOPAD patients was 10.45 years. The mean BMI was 29.84 kg/m². The major cardiovascular risk factors associated with diabetes were dominated by android obesity (87%) followed by hypertension (58.8%). In terms of hypercholesterolemia, LDL levels were elevated in 54.2% and HDL levels were low in 70.8%. 38.9% of our patients were smokers and the number of pack-years was calculated (31.89 ± 32.43 PY). For glycemic control, the mean HbA1c levels of patients with LEOPAD was 8.77 ± 1.78%. LEOPAD was predominantly asymptomatic in our population (88.1%). Only 5 patients (11.9%) had intermittent claudication.
The arterial disease affected the lower left limb in 33.4% of cases, the right lower limb in 45.8% of cases and it was bilateral in 20.8% of cases. Peripheral pulses were diminished or absent in 9 patients (37.5%). In this group, 5 patients (11.9%) had intermittent claudication.
The arteriopathy was compensated in 19 patients, decompensated in 4 patients and at stagechronic critical ischemia in a single patient. Intermittent claudication has been reported by seven patients with an ABI ≤ 0.9. PAD was completely asymptomatic in 70.8% of patients. Among the false negatives (n=17), nine patients had peripheral neuropathy (52.9%) (Table 4).
| Number | Percentage | |
| Compensated LEOPAD | 19 | 79.15% |
| Decompensated LEOPAD | 4 | 16.7% |
| Critical limb ischemia | 1 | 4.15% |
Table 4: Severity of LEOPAD.
The comparative study of patients with LEOPAD showed a statistically conversely significant correlation between LEOPAD and lower levels of HDL (P value = 0.03).However, there is no statistically significant correlation with the following factors: smoking, sex, hypertension, dyslipidemia (total cholesterol and LDL), obesity or overweight android pattern, the presence of microalbuminuria, renal failure and sedentary lifestyle. In terms of glycemiccontrol, it did not show a statistically significant difference between the two groups. Moreover, it was noted that the patients with LEOPAD had had more micro vascular complications compared to patients without LEOPAD. Peripheral diabetic neuropathy was ranked first.
The different correlations between the two groups are summarized in the table 5.
| LEOPAD | Without LEOPAD | P | |
| Meanage | 58 ± 8 ans | 57.31 ± 8.01 | 0.7 |
| Sex ratio | 0.84 | 1.51 | |
| Smoking | 11 (38.9%°) | 49 (45.8%) | 0.33 |
| Sedentary lifestyle | 11 (45.8%) | 32 (25.4%) | 0.117 |
| Onset of diabetes (years) | 10.45 ± 7.18 | 8.04 ± 6.53 | 0.106 |
| Diminished or absent peripheral pulses | 9 (37.5%) | 30 (23.8%) | 0.201 |
| Obesity | 7 (29.2%) | 48 (38.1%) | 0.17 |
| Excessive weight | 12 (50%) | 47 (37.3%) | 0.27 |
| Android obesity | 20 (87%) | 81 (70.4%) | 0.08 |
| Hypertension | 18 (75%) | 76 (60.3%) | 0.12 |
| Hypercholesterolemia | 6 (25%) | 36 (28.6%) | 0.46 |
| Hypertriglyceridemia | 10 (41.7%) | 40 (31.7%) | 0.23 |
| Low HDL | 17 (70.8%) | 60 (47.6%) | 0.03 |
| Elevated LDL | 13 (54.2%) | 84 (66.7%) | 0.17 |
| Hyperuricemia | 3 (13%) | 11 (8.9%) | 0.379 |
| Renal failure | 3 (12.5%) | 16 (12.7%) | 0.64 |
| Positive microalbuminuria | 5 (26.3%) | 33 (30.6%) | 0.47 |
| Positive proteinuria | 4 (16.7%) | 9 (7.6%) | 0.151 |
| CRP : < 1 mg/l 1 – 3 mg/l 3 – 10 mg /l |
1 (4. 16%) 45 (35. 71%) 37 (29. 36%) |
15 (11. 9%) 12 (50%) 7 (29. 16%) |
0.162 0.314 0.401 |
Table 5: Correlations between patients with LEOPAD and patients without LEOPAD.
p: significance rate
In the multivariate analysis. the duration of diabetes as well as the drop
in HDL cholesterol are two predictors of LEOPAD.
In the multivariate analysis, the duration of diabetes as well as the drop in HDL cholesterol are two predictors of LEOPAD (Table 6).
| Variable | Odds Ratio | Confidence interval 95% | P |
| Duration of diabetes | 2.54 | 1.047 - 6.164 | 0.03 |
| HDL-C | 2.67 | 1.036 - 6.887 | 0.03 |
Table 6: Multivariate analysis.
LEOPAD is a marker of systemic atherosclerosis that is associated with high cardiovascular risk complications and cardiovascular death. It is a common disease. However, it remains underestimated because it often remains asymptomatic for a long time [6,7].
The ABI is the ratio of the systolic blood pressure at the ankle divided by systolic pressure at the upper arm. The blood pressure measurement is taken after the patient has been at rest in the supine position for about 10 minutes [8].
The ABI test is non-invasive and inexpensive and is widely used clinically [6] that allows a rapid assessment of the distal lower limb perfusion. ABI decreases when a decrease in limb perfusion occurs. Therefore, ABI is an excellent indicator of lower extremity arterial disease, even before the onset of clinical malfunctions manifest [9]. The the American Diabetes Association (ADA) recommends the assessment of ABI as a first line non-invasive test in patients with symptoms or signs of LEOPAD [10].
The prevalence of LEOPAD in diabetic patients ranges from 2.5 to 16% in Africa [11,12] according to a prospective study involving 308 diabetics recruited from 3 diabetic care centers in the city of Abidjan, the prevalence of LEOPAD was 22.07% [13]. In Tunisia, to the best of our knowledge, our study is the first to provide screening for diabetic arteriopathy by measuring the systolic pressure index and the prevalence in our study was 16% comparable to other results of the literature.
LEOPAD [14,15]. On the contrary, we did not establish that correlation in our diabetic patients. These results were comparable with the konin study made in Abidjan [13]. It is accepted that the longer duration of diabetes causes peripheral arterial lesions that can result in LEOPAD. The resulting atherosclerotic lesions manifest themselves after a variable duration of diabetes onset ranging from 10 to 20 years [16,17]. Our results are consistent with these data of the medical literature because 76.6% of patients with LEOPAD had a duration of diabetes onset greater than 10 years. Sex did not appear to be a determining factor in the occurrence of LEOPAD.
Our results confirm some data from the literature that show a lack of significant difference in sex distribution in diabetic patients with LEOPAD [13,18].
Smoking was encountered in 40% of cases in our patients. This prevalence is less than those reported in the literature. Despite this high proportion of smokers in the patients with LEOPAD, smoking was not related to the occurrence of LEOPAD in our series (P=0.3) which is consistent with the Senegalese authors [18]. In contrast, several other authors have established an association between active smoking and LEOPAD regardless of the geographical variation [11,13,14]. ADA demonstrates that is an independent risk factor for LEOPAD [9].
Obesity is a major risk factor. The type 2 diabetes obesity association is common. The prevalence of obesity among diabetics varies from one study to another in the Western literature with figures ranging from 43% to 52% [19]. In our population of diabetic patients, the prevalence of obesity was 39.3%.
The lipid abnormalities that develop during diabetes have an important role in atherosclerosis. These abnormalities are exacerbated by poor glycemic control. They are characterized by an increase in LDL level that is predictive of atherosclerosis [20].
In our patients, 40.7% were dyslipidemic and hypertension was present in 57.7%. These results are consistent with the konin data [13].
Clinically, the threshold of pain is high in diabetic patients because of the peripheral neuropathy complicating the diabetes. Our study showed consistent results as peripheral diabetic neuropathy was ranked first in microvascular complications in our patients (49.3% of cases). This may explain the lower rate of classic claudication noted in this sample. In the medical literature, some patients with LEOPAD have atypical symptoms as a result of comorbidities, physical inactivity, and alterations in pain perception. Compared with patients with classic claudication, those with leg pain on both exertion and rest were more likely to have diabetes, neuropathy, or spinal stenosis in addition to LEOPAD. Also, there is a discrepancy between the high prevalence of diabetic LEOPAD and the presence of intermittent claudication. The asymptomatic nature of LEOPAD was found in 82.6% to 89.1% of cases in diabetics in various series [22]. Only 12% of our patients had intermittent claudication. Therefore, our results are consistent with the literature.
The high prevalence of LEOPAD in diabetics shows the value of screening for this complication. Measurement of ABI is a simple, noninvasive method of screening for LEOPAD [5,6,21].
There has-been long a controversy over the threshold of the value of ABI used to diagnose an LEOPAD. Its now accepted that the abnormal value to be considered when ABI is <0.90 [22]. The literature demonstrates that diabetic patients with LEOPAD have a significant risk of amputation of the lower limb [15]. ABI<0.90 in diabetic patients is associated with a 7-year risk of amputation if vessels are not revascularized [15]. Our study illustrated a prevalence of 16% of patients with an ABI<0.90. The severity of the LEOPAD was correlated with the ABI value. Thus, the LEOPAD is said to be compensated when an ABI is between 0.75 and 0.90; slightly compensated for an ABI between 0.5 and 0.75. The ABI below 0.50 reflects an LEOPAD with severe impacts.
Some authors confirm that ABI<0.50 is associated with severe arterial lesions demonstrated by angiography [22]. In our series patients with ABI<0.90: 79, 2% (n=19) had compensated arteritis, 16.7% (n = 4) had poorly compensated ABI, and only 1 patient in our series had a severe LEOPAD at the critical ischemia stage, associating an ABI 0.50 and painful symptomatology. This could be attributed to the small size of our sample.
The comparative study of patients presenting LEOPAD versus nonLEOPAD according to the different socio-demographic and clinical parameters showed in multivariate analysis astatistically significant correlation with p=0.03 with the following parameters: low HDL and long duration of diabetes. This can match with the data of the literature [6,14,15].
In fact, the prevalence increases also with rising duration of diabetes as shown in the UK Prospective Diabetes Study (UKPDS): 1.2% at diagnosis of diabetes and 12.5% after 18 years of its evolution [23].
A low concentration of HDL-C is among the strongest lipoprotein risk factors for LEOPAD. In the framingham offspring study, every 5 mg/dL decrease in HDL-C was associated with a 10% increased risk of incident LEOPAD. Similarly, the cardiovascular health study showed a 1% increased odds for every 1 mg/dL decrease in HDL-C [23].
Finally, we point that several studies have demonstrated that the cardiovascular disease and stroke risks were higher in diabetes patients with lower extremity LEOPAD than in diabetes patients without LEOPAD, and lower extremity LEOPAD was an independent risk factor for cardiovascular diseases in diabetes patients. Given that the ABI is a simple and easy method of detecting lower extremity LEOPAD, ABI measurements will be beneficial for the estimation of cardiovascular disease and stroke risks in T2DM patients. The ABI Collaboration conducted a meta-analysis of 16 cohort studies based on individuals, focusing on whether the ABI can predict the risk of cardiovascular events and death independently from the Framingham Risk Score and whether it can improve risk prediction when used in combination with the framingham risk score [5]. The results showed that the use of the ABI would lead to a reclassification of the risk levels for men and women.
We carried out a prospective and descriptive study involved 150 patients with type 2 diabetes which is the first study in Tunisia that was interested screening for diabetic arteriopathy by measuring the pressure index systolic. The main limitation of our study was its monocentric nature with the risks of information and selection bias. It would be interesting to enlarge the workforce and to carry out a multicenter prospective study in order to validate the results obtained on a population other than that of the princeps study.
Lower extremity occlusive peripheral artery disease occurred more commonly than it was expected in the diabetic population, associated with a high cardiovascular comorbidity. The measurement of ABI, which remains largely underused, must be performed routinely in the detection of lower extremity arterial diseases. The sensitivity, specificity and simplicity of the ABI measurement promote its routine use and integration into the clinical approach of high-risk LEOPAD patients, especially the diabetics.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Nacef IB, Khelifi D, Rojbi I, Lakhoua Y, Mchirgui N, et al. (2022) Detection of Lower Extremity Occlusive Peripheral Artery Disease (LEOPAD) by Measuring Systolic Pressure Index in Type 2 Diabetic Patients. J Diab Res Ther 7(1): dx.doi.org/10.16966/2380-5544.159
Copyright: ©2022 Nacef IB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access