Introduction
Obesity and type II diabetes are major health problem in contemporary
society and Indigenous populations are particularly affected [1,2]. For
instance, the prevalence of Type II diabetes is 3 to 5 times higher among
Canadian First Nations living on reserve than in the general urban
population [3]. In some villages, overweight among children also reaches
up to 55% (Canadian government – Healthy weights for healthy kids).
Many hypotheses have been proposed to explain overweight and
obesity in Canadian First Nations: the passage from a nomadic lifestyle
to a sedentary lifestyle, the presence of “thrifty genes” that allow the
accumulation of fat in order to survive and the non-compliance to
drug treatments bringing complications, in addition to allopathic
medicine that is not well adapted to First Nation culture. In order
to address these issues, notably to look toward the traditional
pharmacopeia of the Cree Nations of Eastern James Bay in Canada,
the Canadian Institutes of Health Research Team in Aboriginal Antidiabetic
Medicines (CIHR-TAAM) was founded in 2003. The team
now collaborates with six of the 9 villages and has identified seventeen
plants with anti-diabetic proprieties.
In order to reduce health inequities between general and aboriginal
populations, the CIHR-TAAM studied the anti-diabetic and anti-obesity
proprieties of those seventeen identified plants using a comprehensive
platform of in vitro bioassays and in vivo animal models [4]. One of these
plants, Populus balsamifera (balsam poplar) was found to completely
inhibit the differentiation of 3T3-L1 fibroblasts into mature adipocytes [5].
In subsequent studies, we used the diet-induced obesity (DIO) whereby
C57BL/6 mice fed ad libitum with a high fat diet (HFD) developed insulin
resistance and early diabetes [3]. This was associated with an increase of
total body weight, a heightened profile of blood lipids and a rise in hepatic
steatosis. When DIO mice were fed Populus balsamifera extract along with
the high fat diet, weight gain was decreased as well as hepatic steatosis [3].
Also, glycaemia and insulin levels diminished. Skeletal muscle, liver and
adipose tissue were also probed with several antibodies against various
components of insulin signalling, glucose and lipid metabolism. In these
major insulin target tissues, Populus balsamifera generally improved
insulin-signalling components while favoring components involved in
lipid oxidation in line with the improved insulin sensitivity and reduced
obesity observed systemically.
The intestine is another important organ in lipid homeostasis, being
notably responsible for the packaging and delivery of lipids to the general
circulation [6-8]. Therefore, in the present study, we examined the effect
of Populus balsamifera on the intestinal content of various lipid species
and key protein components involved in lipid metabolism, using tissues
collected during the previous study with DIO mice [3].
Materials and Methods
Plant extracts
Specimens of Populus balsamifera L (Salicaceae family) were collected
in the Cree region of Eeyou Istchee (CEI) located in Northern Quebec,
Canada. A Montreal Botanical Garden taxonomist, Dr. Alain Cuerrier,
confirmed the identity of the plants. A plant sample was deposited in the
Marie-Victorin herbarium of the Montreal Botanical Garden (Mis03-49).
Populus balsamifera (commonly known as balsam poplar) was prepared as
a crude 80% ethanolic extract, as previously described [5].
Animals and diets
Four weeks old and non-diabetic C57BL/6 mice were acquired from
Charles River Laboratories (Saint-Constant, QC, Canada). Mice were
housed in individual cages with lighting schedules of 12 hours (light and
darkness cycles). The rooms were temperature and humidity controlled
and the animals had unlimited access to food and water. As described in
Harbilas [5], mice were divided into groups of a dozen individuals after
having been given time to acclimatize themselves to the animal facilities.
A different diet was given to each of 3 groups of animals. For a period of
16 weeks, the control group (CHOW) received a standard diet composed
of 18% of protein content and 4.5% crude fat, (Charles River Animal Food
and Diet). For a period of 8 weeks, the 2 other groups received a high fat
diet (HFD, Bio-Serv Diet #F3282, 60% energy from fat). Following this
period, one of these groups continued the high fat diet for another 8 weeks
while the other group received HFD into which Populus balsamifera
extract was incorporated at 125 mg/kg, this being the most active dose
seen in previous studies [3]. The Université de Montréal Ethic Committee
approved all experimental protocols and protocols followed the guidelines
from the Canadian Council for the Care and Protection of Animals.
Surgical procedure
Once the experimental protocol ended, an intra-peritoneal injection
of 50 mg/kg pentobarbital was used to anesthetize mice and then animals
were sacrificed by ex-sanguination. Intestinal samples were collected
rapidly thereafter, rinsed with PBS containing a mixture of anti-proteases
(50 mM sodium fluoride, 1 mM phenyl methyl sulfonyl fluoride, 5 µg/ml
and aprotenin, 5 µg/ml leupeptin).
Lipid analyses
Aliquots of jejunal homogenates were lipid-extracted with 2:1 (vol/
vol) chloroform- methanol [9,10]. Small amounts of lipid standards were
added to the samples before the separation of individual lipid classes
by one-dimensional thin layer chromatography (TLC) (silica gel from
Eastman Kodak, Rochester, NY) as described previously [10,11]. The
non polar solvent system was 80:20:3 (vol/vol/vol) hexane-diethyletherglacial
acetic acid. The lipid concentrations of the separated fractions were
measured enzymatically by commercial kits (Boehringer Mannheim,
Montreal) after elution with exan and evaporation.
Western blot analysis
To assess the protein expression of various proteins, the jejunal tissue
was homogenized in lysis buffer [50 mM HEPES (pH 7.5), 150 mM sodium
chloride, 1.5 mM magnesium chloride, 1 mM EGTA, 1% TritonX-100, 50
mM sodium fluoride, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride,
5 µg/ml Aprotenin, 5 µg/ml leupeptin, and 1 mM sodium orthovanadate],
followed by centrifugation at 13,000 rpm for 10 min and prepared for
Western blotting as described previously [12]. The following antibodies
were employed: anti-FAS, anti ACC, anti-pACC anti-CPT1 from Santa
Cruz Biotechnology (Santa Cruz, CA).
The Bradford assay [Bio-Rad Laboratories (Canada) Ltd., Mississauga,
Ontario, Canada] was used to determine protein concentration. Proteins
were denatured in sample buffer containing SDS and β-mercaptoethanol,
separated on a 7.5% SDS-PAGE gel, and blotted onto nitrocellulose
membranes. Nonspecific binding sites of the membranes were blocked
using 5% defatted milk proteins. Reactions took place by the addition
of primary antibodies directed against targeted proteins. Reaction
was revealed with species-specific horseradish peroxidase- conjugated
secondary antibody and enhanced chemiluminescence reagent
(PerkinElmer, Waltham, MA). β-actin was used as an internal control to
confirm equal loading protein on SDS-PAGE. Blots were developed, and
proteins were quantified using a Hewlett-Packard scanner equipped with
a transparency adaptor and UN-SCAN-IT (Silk Scientific Inc., Orem, UT)
software.
Statistical analysis
A one-way analysis of variance (ANOVA) was used to analyze data
using the software Prism 5.0. Statistical significance was set at p<0.05.
Results
Intestinal lipid parameters were measured in jejunal segments obtained
from CHOW controls, DIO controls (16 w eeks on HFD) and DIO animals
receiving 125 mg/kg of Populus balsamifera extract for the last 8 weeks
of HFD treatment. As illustrated in figure 1, intestinal total cholesterol
content was not affected by the DIO treatment in the absence or presence
of Populus balsamifera extract. Similar results were obtained for jejunal
phospholipids (Figure 2).
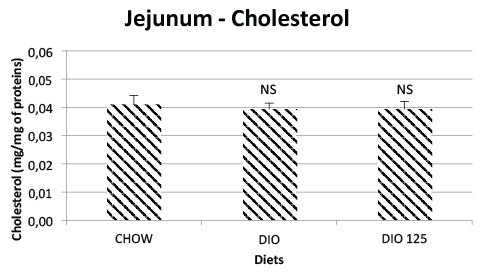
Figure 1: Jejunal total cholesterol is not modified by high fat diet or
P. Balsamifera treatment. Jejunal total cholesterol was measured as
described in Materials and Methods and expressed as mg per mg of
jejunal protein. N.S. Not significantly different from CHOW controls by
one-way AVOVA, N=8 per group.
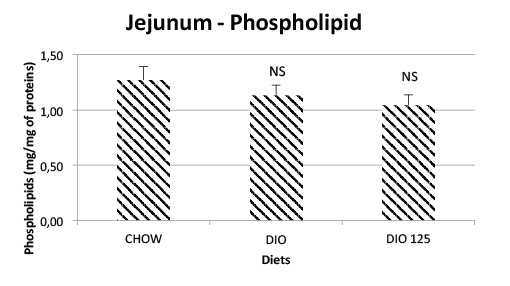
Figure 2: Lack of effect of high fat diet or P. Balsamifera treatment
on jejunal phospholipid content. Jejunal phospholipid content was
determined as described in Materials and Methods and is expressed as
mg per mg of jejunal protein. N.S. Not significantly different from CHOW
controls by one-way ANOVA, N=8 per group.
In the case of intestinal triglycerides, there was a tendency of increase
with the 16-week feeding of a HFD and this trend seemed to vane with
Populus balsamifera treatment (Figure 3). However, data variability
precluded any statistical significance in these trends. On the other and,
the jejunal content in fatty acids was significantly increased by the DIO
treatment as compared to CHOW controls (p<0.05; Figure 4). Populus
balsamifera treatment significantly reduced intestinal fatty acid content
back toward values observed in CHOW controls (p<0.05 versus DIO, N.S.
versus CHOW; (Figure 4).
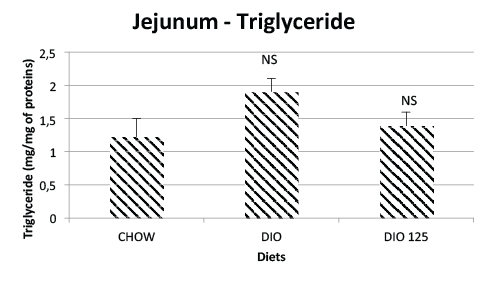
Figure 3: Jejunal triglycerides are not significantly affected by high fat
diet or P. Balsamifera treatment. Jejunal triglycerides were measured
as described in Materials and Methods and expressed as mg per mg of
jejunal protein. N.S. Not significantly different from CHOW controls or
from DIO group by one-way AVOVA, N=8 per group.
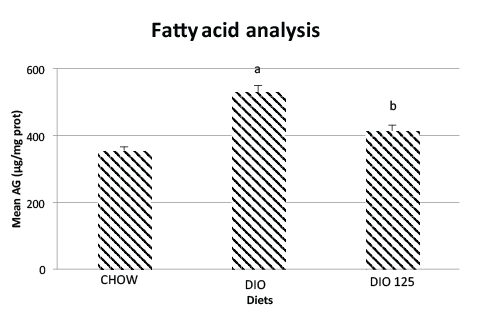
Figure 4: High fat diet increases jejunal fatty acid content and this effect
is reversed by P. Balsamifera treatment. Jejunal fatty acid content was
measured as described in Materials and Methods and is expressed as
µg per mg of jejunal protein. Significantly different from CHOW controls
(a; p<0.05) or from DIO group (b; p<0.05) by one-way ANOVA, N=8 per
group.
To elucidate the mechanisms underlying changes in intestinal lipid
handling, we used Western blot analysis to measure the expression of
three key lipid metabolizing enzymes, namely fatty acid synthase (FAS),
carnitine palymitoyltransferase-1 (CPT-1) and acetyl co-Acarboxylase
(ACC). As shown in figure 5, the DIO dietary regimen induced a significant
decrease in the content of intestinal FAS (p<0.05 versus CHOW) and
Populus balsamifera treatment had a tendency to partially normalize
the values (N.S. versus either CHOW or DIO control groups). On the
other hand, CPT-1 was reduced in both DIO and Populus balsamiferatreated
mice as compared to CHOW control animals (p<0.05; (Figure 6).
In the case of the phosphorylated (inactivated) form of ACC (pACC), it
was again significantly diminished by the DIO regimen (p<0.05 versus
CHOW; (Figure 7). However, Populus balsamifera treatment completely
returned levels of pACC back to levels found in CHOW control mice (N.S.
versus CHOW, (Figure 7).
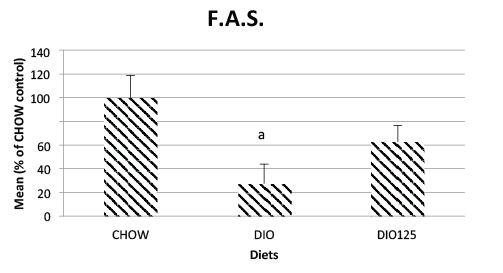
Figure 5: Jejunal fatty acid synthase is reduced by high fat diet treatment
but not when the latter is combined with P. Balsamifera treatment. Jejunal
FAS was assayed by Western blot analysis as described in Materials
and Methods and is expressed as a percentage of densitrometric values
obtained for CHOW control animals. Significantly different from CHOW
controls (a; p<0.05) by one-way ANOVA. N=8 per group.
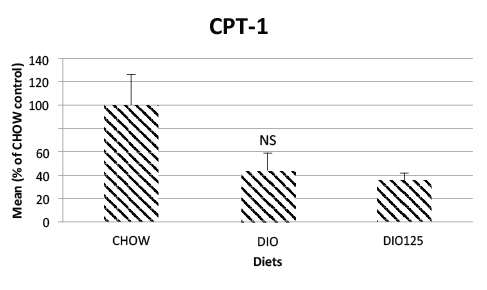
Figure 6: Jejunal CPT-1 is not significantly affected by high fat diet or
P. Balsamifera treatment. Jejunal CPT-1 were measured by Western
blot analysis as described in Materials and Methods and is expressed
as a percentage of densitrometric values obtained for CHOW control
animals. N.S. Not significantly different from CHOW controls or from
DIO group by one-way AVOVA, N=8 per group.
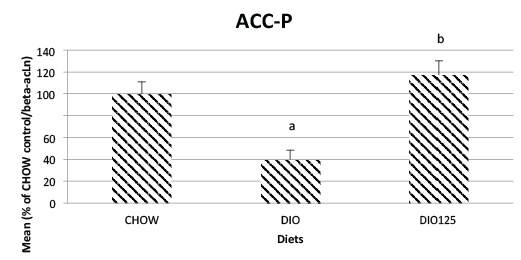
Figure 7: High fat diet decreases jejunal phosphorylated ACC and this
effect is reversed by P. Balsamifera treatment. Jejunal phosphorylated
ACC was measured by Western blot analysis as described in Materials
and Methods and is expressed as a percentage of densitrometric values
obtained for CHOW control animals. Significantly different from CHOW
controls (a; p<0.05) or from DIO group (b; p<0.05) by one-way ANOVA,
N=8 per group.
Discussion
Our team, the CIHR-TAAM, has been working since 2003 with the
Cree First Nations of Eastern James Bay in Canada to rigorously study
potential anti-diabetic and anti-obesity plants from their traditional
pharmacopeia. While screening for glitazone-like activity in differentiating
3T3-L1 adiopcytes, we discovered that the ethanol extract of balsam
poplar, Populus balsamifera, completely abrogated adipogenesis, thereby
suggesting a possible anti-obesity effect [5,13]. We have thus more recently
carried out an in vivo study using the DIO mouse model to examine the
effects of balsam poplar on body weight and metabolic parameters. We
reported that blood lipid parameters of DIO mice were modulated in the
following manner: LDL levels were more than tripled, HDL increased by
about 50% and total cholesterol doubled as compared to CHOW controls,
whereas circulating triglycerides remained unchanged [3]. Although it
reduced body weight and improved glucose homeostasis as well as insulin
sensitivity, Populus balsamifera treatment did not have any significant
effect on blood lipid parameters. However, it reduced hepatic steatosis
and corresponding liver triglyceride content as compared to DIO control
animals [3].The intestine is another important organ implicated in body
lipid homeostasis [14]. Since we had also collected intestinal samples at
animal sacrifice in our previous work [3], the aim of the current study
was to evaluate the effects of the DIO protocol and of Populus balsamifera
treatment on jejunal lipid content and on key proteins implicated in
intestinal lipid homeostasis.
The results presented herein demonstrate that the DIO regimen of
feeding a HFD for 16 weeks had little effect on jejunal total cholesterol and
phospholipids while it tended to elevate intestinal triglycerides, albeit not
in a statistically significant manner. In contrast, the feeding of the HFD
increased jejunal fatty acid content significantly.
This effect was reversed by Populus balsamifera treatment while other
lipid parameters were not significantly affected, although there was a trend
for the plant to reverse the DIO-induced reduction of FAS expression.
Together with our previously reported results on systemic lipid parameters
in DIO mice [3], the present study suggests that despite a large dietary lipid
load, little cholesterol, phospholipids and triglycerides seem to accumulate
in intestinal cells. In contrast, the increase in jejunal fatty acids may
stem from an overload of the lipid producing machinery of enterocytes.
Indeed, our results showed that the expression of FAS was diminished in
DIO animals. This would limit fatty acid synthesis in accordance with the
large dietary supply of the latter. Similarly, the expression of CPT-1, a key
enzyme of lipid beta-oxidation, was also reduced. This would limit the
oxidation of fatty acids and contribute further to their observed cellular
accumulation. On the other hand, ACC phosphorylation (pACC) was
decreased in DIO animals and this indicates an increase in ACC activity.
Normally, this should supply more malonyl-CoA for fatty acid synthesis,
but FAS was found to be diminished. Malonyl-CoA also inhibits CPT-
1, which is consistent with our current observations. One possibility to
explain the reduced pACC is that jejunal AMP-dependent kinase activity,
which normally phosphorylates and inhibits ACC, may be diminished in
DIO animals. However, the phosphorylated and active form of AMPK
in skeletal muscle was not significantly different between DIO mice
and CHOW controls in our previous work [3]. Further studies will be
necessary to clarify this point.
Without affecting circulating lipid parameters in DIO mice, we
previously reported that Populus balsamifera treatment reduced body
weight, improved glycemia and insulinemia while favouring insulin
signalling and fat oxidative components in liver and adipose tissue. In
jejuna segments in the current study, Populus balsamifera treatment had
two major effects. It reduced the cellular fatty acid content and increased
ACC phosphorylation. Both of these components could be related to
an increase in AMPK activity leading to lipid “wastage”, as previously
suggested [3]. Indeed, we have also previously reported that balsam
poplar more than doubled AMPK activation in cultured hepatocytes
[15]. However, since the reduced expression of CPT-1 was not
reversed by Populus balsamifera treatment, it is hard to envisage that
beta oxidation of fatty acids may have been improved. Other oxidative
pathways, such as omega-oxidation of fatty acids, could be increased in
the intestine, as was seen with dietary lipid interventions in non-obese
C57BL/6J mice [16].
Conclusion
In summary, the effects of Populus balsamifera treatment on jejunal
segments of DIO mice were limited to reducing fatty acid content and
ACC activation (through increased phosphorylation). Nonetheless, such
actions should be beneficial in the face of high fat diets and obesity. This
further strengthens the potential of balsam poplar extracts to be useful in
the context of the high prevalence of obesity in Indigenous populations,
such as the Cree of Eeyou Istchee. Populus balsamifera traditional
preparations should thus be studied further, notably in clinical settings,
in such populations.
Acknowledgements
These studies were funded by a Team Grant from the Canadian Institutes
of Health Research (CTP-79855) to PSH. Jonathan Ferrier (laboratory of
John T. Arnason, University of Ottawa) is gratefully acknowledged for
preparing the crude extracts of Populus balsamifera.
Very special thanks are due to Smally Petawabano, Laurie Petawabano,
Charlie Coon, Sophie Coon, Mabel Gunner, Abel Mark, Kathleen Mark,
Elizabeth Coon Come, Harriett Matoush, Sandy Matoush, Rene Coon,
and Emma Coon Come from the Cree Nation of Mistissini, and Abraham
Mamianskum, Agnes Kawapit, Andrew Natachequan, Anne Sandy, Eliza
George Mamianskum, Eliza Kawapit, James Kawapit, Jeanny Masty,
John Petagumskum Sr., Lucy Rupert, Maggie Natachequan, and Mathew
Natachequan from Whapmagoostui First nation, as well as to 41 other
Cree Elders of both nations who kindly agreed to be interviewed. They
made this article possible by allowing us to use, for the purposes of this
research, their knowledge relating to medicinal plants transmitted to them
by their Elders. Their trust has also enabled a useful exchange between
Indigenous knowledge and Western science.
Disclosure
The authors declare no conflict of interest.








