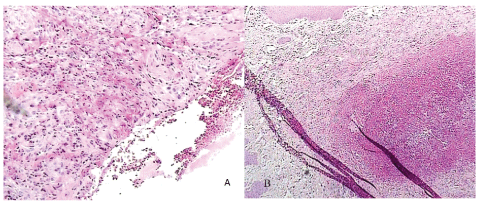
Figure 1: Comparison between histopathological examination of suture A (a) and control suture (b) in day 4 (hematoxylin and eosin staining with ×100 magnification)


Majid Zakeri1* Nooshin Arjmand2 Ali Forouzanfar3 Mahdi Zakeri4 Niloufar Koohestanian5
1General dentist, Mashhad, Iran*Corresponding author: Majid Zakeri, General dentist, Mashhad University of Medical Sciences (MUMS), Vakil-Abad Boulevard, Mashhad, Iran, Tel: +98(915)322-3703; E-mail: majid_dentist85@yahoo.com
Background: It has been postulated that conventional suture materials such as silk may enhance bacterial biofilm growth causing delay in healing of surgical sites. Nano-silver particles (NS) with their anti-bacterial agent properties could be helpful. This study evaluates the role of Nano-silver particles on the inflammatory process of gingival suture in comparison with usual silk suture in animal model.
Materials and methods: In this double-blind clinical random study, 12 female rabbits with normal teeth and no periodontal disease had been found by clinical and radiographic examinations were selected. Our innovated Nano-silk suture, Nano-silver particles coated on the surface of conventional silk suture in specific environment, was prepared in sterile condition and under special circumstances with Nano-silver particles. Two different density of Nano-silk (A and B) were prepared. A suture contained 60 ng of silver and B sutures included 120 ng of silver. Nano-silk sutures thread and conventional silk suture thread were sutured on the buccal surface of the gingiva of mandibular incisors. Histological changes on 4th and 7thsday after suturing were evaluated to assess the inflammatory process.
Results: The histological results showed that the application of this new suture material may improve inflammation process and promote wound healing. We also observed a relatively marked difference between the two groups in the 4th and 7th days in the inflammatory cell’s components and edema. These two parameters are important factors in the wound inflammation process. Our results suggested that the density of Nano-silver particles in Nano-silk B causes less inflammatory response in comparison with Nano-silk A and therefore a better scaffold for parenchymal and mesenchymal factors is made one week after surgery
Conclusion: Silver particles due to their beneficial effects as antibacterial and wound healing accelerator in the periodontal surgeries could reduce inflammation and promote healing process. So Nano-silver sutures could be helpful for and produce a more favorable healing when used in oral and maxillofacial surgery.
Nano-silver particles (NS); Suture; Periodontal flaps; Necrosis; Edema; Inflammation; Gingivectomy
Recently the Nano-silver particles (NS) coated on conventional silk stitch as the new emerging technique in the nanotechnology industry have been the fastest growing products. Its antimicrobial feature, led to increasing number of its medical applications. Some of such products already available in the market include wound dressings, contraceptive devices, surgical instruments and bone prostheses [1-6].
The term ‘‘Nano-Silver’’ refers to the nanoparticles of silver with a size between 5-50 nm. The active surface of Nano-Silver is 4 m2/g compared to 1-2 m2/g of commercial silver powder [7]. During the chemical reduction, the reducing agent donates electrons to the silver ions (Ag+), causing silver to revert to its metallic form (Ag0 ). By controlling the experimental conditions including the temperature, energy input, presence of capping agents, the reaction kinetics could be influenced including the clustering silver atoms form NS of Nano scale dimensions [7-9].
Previous evidences suggested that NS has a potent anti-inflammatory effect [4–6] and accelerates wound healing [7,8]. Also, recent study by Shahoon et al. [10] confirmed that the differences in level of inflammation and presence of necrosis were significant in Transforming Growth Factor Beta (TGFβ) with Nano-silver groups vs TGF ones. They concluded that the detection of chondrogenesis in one sample of subperiosteal rabbit parietal bone can be explained by decreased Nano-silver dose and difference in anatomic site of injection.
Oral cavity is a normal habitat for several bacterial species, many of which are capable of causing acute and chronic infection if any cut or wound appears on the epithelium. Patients with Immuno deficiencies and cancerous patients, due to weak immune system experienced more delay in their wound healing. Improving wound healing and decreasing the microbial flora enhance defense system so antiseptic materials for periodontal surgeries and oral wounds have received important attention. Regenerative, respective surgeries, oral implants and reconstructive dentistry, without controlling the microbial activity will fail [11]. Recent studies have been revealed antiseptic properties of Nano-silver particles [9,12,13] in burn wound regeneration [14-16], diabetic foot patients [15- 18] and mucositis [19]. They had a protective effect in cellular proliferation and viability of wounds [20]. Also application of the Nano-silver particles with bone cement had positive effects in preventing resistant infections and increased success of joint arthroplasty surgery [21,22].
In this study, we examined the effects of Nano-silver suture for healing of periodontal flaps in rabbits and evaluated some parameters including the inflammation, edema and necrosis by histopathological assay after rabbit’s gingivectomy.
Manufacturing company performed the following procedure to manufacture the Nano-silver sutures in the recovery stage.
Nano-silver particles were ionized between two positive and negative electrodes in vacuumed container and the suture silk between two electrodes were covered. The strength of antimicrobial activity of this application is related to the Ag+ which flow between two electrodes. Through increasing the flow, the Nano particle size, on the silk surface of suture was increased. In this process, the temperature must be controlled because high heat diverges Nano particles and covered container coverage instead of suture surface. All procedures should be repeated under standard laboratory situations. After completing the processes, these following tests were performed on Nano-silver sutures:
Twelve female adult rabbits, which were supervised by ethic committee of Mashhad university of Medical Sciences (MUMS) and we had gotten their official permission to perform this animal study under ethical and laws protocol, underwent to equal vaccination and placed in standard situations through similar nourishment for the aim of this study. All animal subjects were healthy with normal teeth and no periodontal disease had been found by clinical and radiographic examinations and also did not have any underlying diseases. The rabbits were randomly divided into two groups of six rabbits each; group A and group B with different therapy dosages. Subsequently each group divided into two subgroups as control and related therapy. Gingivectomy had been planned for each rabbit to involve the bilateral mandibular teeth.
This study was double blind as the researchers and the consultant pathologist were not aware of Nano-silver dosage in order to prevent any manipulation by either researchers or pathologist. Only the representative of Nano-silver manufacturer Company was aware of study groups and dosage that informed us after evaluation of samples by pathologist to figure out which group was prominent in this study. For all twelve studied rabbits, two sutures were taken, in lower gingiva of two left and right front teeth. One suture with simple silk as control suture was examined for all rabbits. While the other suture performed by Nano-silver silk that had different density depending on the group A or B. Following the surgery, the periodontal dressing (Coe-Pak, GC America INC) was placed on both experimental and control sides.
For histological examination, we prepared two biopsies on postoperative day 4 and 7. Two 5 mm × 5 mm with 2 mm depth biopsies with healthy tissue around the wound were taken, one Nano-silver suture and one control suture. The biopsy samples were placed in 10% neutral buffered formalin for evaluation. After fixation, the segments were embedded in paraffin and sectioned at 4 µm thickness. The samples with unsuitable fixation were excluded and just the samples with suitable size and thickness were included.
Then the samples were stained with hematoxylin and eosin and examined with light microscopy by pathologist who was blinded about study procedure. The parameters used for evaluation were cellular components including neutrophils, lymphocytes, plasma cells, macrophages, eosinophils and mast cells, necrosis and edema.
To evaluate inflammation density, each of the parameters was scored from 0 to 3 as follow: 0=normal, 1=mild increase (in inflammation cells), 2=moderate increase and 3=marked increase. For evaluation of inflammatory cell density, few and random arrangement of all inflammatory cell types; including acute inflammation cells (like neutrophils), chronic inflammation cells (like lymphocyte), plasma cells and macrophages in dermis were considered normal. Furthermore, presence of 3-10, 11-30 and 31 or more cells in wound tissue per × 400 magnification fields was considered as mild, moderated and marked increase respectively.
To evaluate necrosis, sparsely scattered necrotic cell debris and extraverted erythrocytes were considered as mild necrosis and focal dense accumulation of each of these components in the wound tissue were considered as moderate necrosis. The marked necrosis considered as the extensive tissue necrosis, massive hemorrhage involving surrounding tissue and the presence of greater than 30 macrophages per × 400 magnification fields.
For edema evaluation, the scant collagen bundles which slightly separate fibroblasts in the wound tissue were considered as a mild increase in collagen density. Extensive separation of fibroblasts by abundant collagen was considered a marked increase in collagen density. Slight separation of cells and collagen from one another in the wound tissue by non-staining or poorly staining a cellular material was considered as a mild edema. Moderate edema and intensive edema were considered as 30 to 50 µm and greater than 50 µm separation of a cellular material respectively.
Mean scores were used for objective evaluation of histological assessments. The data were statistically analyzed by Mann-Whitney test between studied groups. Also Wilcoxon test was performed for comparison of groups in days 4 and 7 after surgery. A p-value less than 0.05 were considered significant. SPSS for Windows, version 16 (SPSS Inc., Chicago, IL, USA) was used in all statistical procedures.
We evaluated some factors including necrosis, cellular inflammatory components and edema in days 4 and 7 after surgery. The pathology figures showed that Nano-silver suture A and B were effective in reducing inflammatory responses compared to control sides. However, there were not any statistically significant differences between two studied groups and controls. This point might be attributed to low sample size or low power of study.
Table 1 show that control group and group A had the same severity of edema in day four. Analysis of the density of inflammatory cell components showed that in group A suture, rate of edema with moderate severity was higher than edema with marked severity compared with control sides, although difference was not significant (P>0.05). Also mild necrosis was more frequent than marked necrosis in group A suture compared with control side. Figures 1A and 1B showed that for group A, mild to moderate inflammatory infiltration of cellular components, growth through movement of epithelial gingival tissue to wound edges and consequently regeneration stimulation of epithelial basal layer cells and formation of frequent vascular buds, were occurred. While in control side, the sever infiltration to gingival epithelial tissue, edema and necrosis were not observed. All inflammatory parameters in group A was decreased in day 7th postoperative compared with day 4th and control sides (Table 1). Histopathological findings showed that wound edge was completely separated from each other, besides the fibrinoleukocytic detritus as pseudo membrane covered the wound surface in control group. This condition is not a suitable base for regeneration due to the remained inflammatory mediators and necrotic materials (Figures 2A and 2B).
|
|
Day 4 |
Day 7 |
||||
|
Severity |
Cellular components |
Edema |
Necrosis |
Cellular components |
Edema |
Necrosis |
Group A |
Normal |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
3 (100) |
Mild Moderate |
1 (33.3) |
1 (33.3) |
3 (100) |
3 (100) |
3 (100) |
0 (0) |
|
Marked |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
|
Control |
Normal |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
1 (33.3) |
Mild Moderate |
1 (33.3) |
1 (33.3) |
1 (33.3) |
0 (0) |
2 (66.7) |
2 (66.7) |
|
Marked |
1 (33.3) |
0 (0) |
0 (0) |
1 (33.3) |
0 (0) |
0 (0) |
|
P- value |
0.65 |
1 |
0.15 |
0.15 |
0.3 |
0.15 |
|
Table 1: Comparison of cellular components, edema and necrosis in group A and control on day 4 and day 7 P-value less than 0.05 considered significant.

Figure 1: Comparison between histopathological examination of suture A (a) and control suture (b) in day 4 (hematoxylin and eosin staining with ×100 magnification)
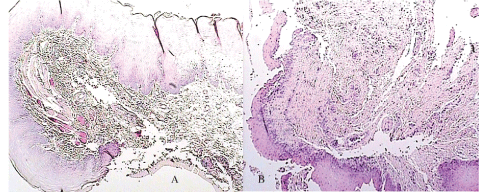
Figure 2: Comparison between histopathological examination of suture A (a) and control suture (b) in day 7 (hematoxylin and eosin staining with × 100 magnification)
Table 2 showed that there was not any difference between cellular components, and edema of suture B and control suture in day 4, but necrosis was high in suture B group. Histological assay of Nano-silver in group B in day 4 presented the moderate inflammatory cells infiltration and mild to moderate edema besides different vascular buds through new formed collagen fibers (Figure 3A). All inflammatory parameters were decreased in control sides compared to Nano-silver B suture in day 7 (Table 2). Also mild edemas, inflammatory cell density besides complete epithelization in wound edge were showed in figure 3B.
|
|
Day 4 |
Day 7 |
||||
|
Severity |
Cellular components |
Edema |
Necrosis |
Cellular components |
Edema |
Necrosis |
Group B |
Normal |
0 (0) |
0 (0) |
0 (0) |
1 (33.3) |
0 (0) |
1 (33.3) |
Mild Moderate |
0 (0) |
0 (0) |
1 (33.3) |
2 (66.7) |
3 (100) |
2 (66.7) |
|
Marked |
1 (33.3) |
0 (0) |
1 (33.3) |
0 (0) |
0 (0) |
0 (0) |
|
Control |
Normal |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
Mild Moderate |
0 (0) |
0 (0) |
1 (33.3) |
2 (66.7) |
2 (66.7) |
3 (100) |
|
Marked |
2 (66.7) |
1 (33.3) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
|
P- value |
0.56 |
0.31 |
0.65 |
0.15 |
0.31 |
0.31 |
|
Table 2: Comparison of cellular components, edema and necrosis in Nano-silver group B and control on day 4 and day 7 P-value less than 0.05 considered significant.
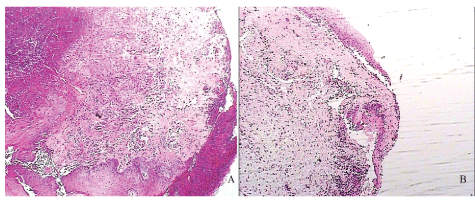
Figure 3: Histopathological view of suture B in day 4(a) and day 7 (b); Arrows pointed the new formed vascular buds and NCO is abbreviation of new collagen fiber (hematoxylin and eosin staining with × 100 magnification).
However similar edema was observed in two studied groups in day 7, but cellular components density and necrosis were higher in group B than group A (Table 3).
|
|
Day 4 |
Day 7 |
||||
|
Severity |
Cellular components |
Edema |
Necrosis |
Cellular components |
Edema |
Necrosis |
Group A |
Normal |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
3 (100) |
Mild Moderate |
1 (33.3) |
1 (33.3) |
3 (100) |
3 (100) |
3 (100) |
0 (0) |
|
Marked |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
|
group B |
Normal |
0 (0) |
0 (0) |
0 (0) |
1 (33.3) |
0 (0) |
1 (33.3) |
Mild Moderate |
0 (0) |
0 (0) |
1 (33.3) |
2 (66.7) |
3 (100) |
2 (66.7) |
|
Marked |
1 (33.3) |
0 (0) |
1 (33.3) |
0 (0) |
0 (0) |
0 (0) |
|
P- value |
0.40 |
0.76 |
0.2 |
0.7 |
1 |
0.2 |
|
Table 3: Comparison of cellular components, edema and necrosis in Nano-silver group A and Nano-silver group B on day 4 and day 7 P-value less than 0.05 considered significant.
Recently medical aspects of Nano-silver particles including antibacterial properties, wound healing and anti-inflammatory effects have been showed. Some studies reported that Nano-silver therapy is safe without side effects at dosages lower than 10 mg/kg, while it is toxic at dosages more than 20 mg/kg [23]. Studies also reported that it’s healing and side effects properties are time dependent, as favorable biological effects were observed after 7 and 14 days while marked and toxic inflammation was observed after 30 days [24].
Oral Wound regeneration compared to other sites has unique problems due to high number of bacteria and frequent irritation by chewing and speaking. Therefore, the biomaterial suture with antibacterial properties besides accelerating wound healing process agents is necessary to deal with both bacteria and local irritations. Secondary infection of sutures due bacterial biofilm formation in hospitals and clinical centers is seen frequently [25,26]. Nano-silver sutures by eliminating the sutures bacterial biofilms may decrease the tissue inflammatory processes and expedite the tissue regeneration process. In this study Nano-silver suture had different dosage and it was under manufacturer right until the results were obtained. At the end of study, we found that Nano-silver suture A and B had 60 ng and 120 ng silver respectively.
Our results showed that severity of cellular components was slightly higher in group B than group A in day 4. Besides our study results showed that edema and necrosis were higher in control sites than group A and group B. Severe increase of inflammatory cells, especially during the first days, accelerate cleaning of damaged cells and necrotic remains. On the other hand, it could cause cytokine secretion and procuring the regeneration process. Also comparison of the cellular components density between suture A, suture B and control side showed that it was greater at control group in day 7. This finding was similar to Wrightet al. [27] study as they found that healing was characterized by rapid development of well vascularized granulation tissue that supported tissue grafting 4 days’ post-injury, unlike control dressed wounds. The results suggested that silver component dressing altered or compressed the inflammatory events in the wounds and facilitate the early phases of wound healing. However, they concluded that these results were associated with reduced local matrix metalloproteinase levels and enhanced cellular apoptosis. Nonetheless we didn’t measure the metalloproteinase activity, but Woodward [28] showed that silver ions caused neutrophils apoptosis and consequently suppress the inflammatory events.
Inflammatory factors have been similar in group A and group B in day 4, but inflammatory parameters in group B was decreased in day 7,so that decreasing the rate of inflammatory processes for suture B was greater compared to suture A. This could have facilitated synthesis of collagen scaffold, synthesis of connective tissue components and the other regeneration factors. Longer inflammatory processes in A-suture compared to B-suture, is due to its lower concentration of Nano-silver particles. In group A suture, Although Nano-silver particles facilitate wound healing, its effectiveness is probably correlated to an optimum concentration. Higher silver particles act as foreign bodies, exert mild toxic effects and cause continuing of inflammatory responses. Also, Poon et al. [29] assessed the effects of culture environment on the susceptibility of the cells by the toxic action of silver. They concluded that cytotoxic effects of silver and silver-based products must be considered when deciding to use them for dressings of specific wound care. This is important when using keratinocyte culture, in situ, which is playing an increasing role in contemporary wound and burn care.
We might conclude that silver particles have beneficial effects as antibacterial and accelerate wound healing in the periodontal treatments. In addition, the Nano-silver particles in this suture may promote the treatment results of the surgery. Moreover, its clinical application can reduce the inflammation, improve healing process and reduce scar in all of gingival, oral and maxillofacial surgery. On the other hand, surgery sites interrupted by rabbit’s hand intervention at mealtime and sometimes they completely destroyed control and Nano-silver dressing, forced us to perform all study protocol in each animal again and also it impeded our progress several times. It seems clear that choosing appropriate animal for this aim could prevent such troubles in further studies. Further researches is warranted to further elucidate the optimal dosage for their application in suturing of oral surgical wounds and also evaluate future implications of Nano-silver particles such as bacterial count along soft tissue.
Download Provisional PDF Here
Article Type: Research Article
Citation: Zakeri M, Arjmand N, Forouzanfar A, Zakeri M, Koohestanian N (2016) Nano-silver Suture as a New Application for Healing of Periodontal Flaps. Int J Dent Oral Health 2(7): doi http://dx.doi.org/10.16966/2378-7090.217
Copyright: © 2016 Zakeri M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access