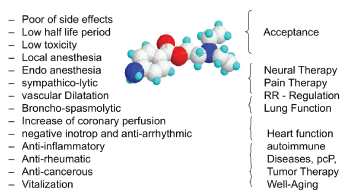
Figure 1: Overview of the most important pharmacological and clinical features of Procaine.

Uwe R M Reuter1 Ralf Oettmeier2* Huseyin Nazlikul3
1Medical Director of the Hospital Klinik im Leben, Vogtland, Germany*Corresponding author: Ralf Oettmeier, Head Physician of the Alpstein Clinic in Gais, Switzerland, E-mail: ralf.oettmeier@icloud.com
The highly-dosed infusion with Procaine-HCl with sodium-bicarbonate as additive was firstly published twenty years ago. The method advanced to a routine in many centers for pain treatment, rehabilitation and natural medicine. The aim of the procedure is the systemic use of the various pharmacological features of Procaine, especially to inhibit pain and inflammation, for vasodilatation, anti-oxidation and to harmonize the vegetative nervous system. The addition of sodium-bicarbonate balances the common latent pH-decrease in the periphery. The degradation products of Procaine, diethylaminoethanol (DEAE) and para-amino benzoic acid (PABA), have a systemic effect. For the safety of the patients after 500.000 applications: the procaine-infusion is safe. To improve the success rate of the method of the classic Procaine- Base-infusion should be realized an acid-base-diagnostic.
Procaine; Safety of procaine; Inflammation; Infusion; Pain; Rheumatism; Sodium bicarbonate; Neural therapy
Procaine was originally created in 1905, as the original man-made local anesthetic by a German chemist, Alfred Einhorn (1857-1917). Unlike novocaine (procaine with a sulphite preservative) it doesn’t cause allergic reactions in patients. Procaine stabilizes membranes of nerves, sympathetic nerves, and mast cells. It increases vasodilatation and is easily metabolizes in the plasma by the enzyme Pseudo cholinesterase through hydrolysis into para-amino benzoic acid (PABA) and diethylaminoethanol (DEAE). It has a bitter taste and a half-life of 15-20 minutes, which is brief. It is a safe medicine and has been used in famous anti-aging clinics such as Dr. Ana Aslan’s clinic in Romania where she treated Bob Hope, Cary Grant, Marilyn Monroe, Winston Churchill and others [1].
The local anesthetic Procaine is characterized by a sum of pharmaceutical features. With this in mind Prof. ASLAN, the founder of the eponymous therapy, spoke of it as vitamin-like action beside the anesthetic effects [1]. Further benefits of Procaine are its good tractability and low-grade toxicity due to its short half-life and plasma degradation, the capillary impermeability effect [1], the inhibition of inflammation [1-4], anti-oxidative and fat-reducing action [5-7]. Contrary to all other anesthetic drugs it causes vasodilatation of vessels and capillaries [8-13]. With this therapy it is possible to reach and optimally influence very poorly circulated tissue (especially in case of inflammation and pain). Beside the effect of blocking voltage-dependent sodium channels with the result of a short-term anesthesia [14], additional actions of Procaine on cell membranes and the matrix as well as sympatholytic actions were also discussed [15-20]. Krause has demonstrated that the anti-inflammatory effect of Procaine in rheumatic disease was especially high when combined with an alkali additive [7]. In the field of oncology, the effect of Procaine to reduce side effects from radiotherapy [21,22] or to improve the influence of chemotherapy [23-26] is reported. The epigenetic action of the procaine and the growth-inhibition after incubation with human cancer cells due to the partial blockade of DNA-methylase in vitro was described in 2003 [27]. A diminishing effect of the proportion of 5-methylcytosine into global genomic DNA and cell proliferation due to procaine was reported in a study of tumor suppressor genes [28]. The inhibition of DNA methylation in human hepatoma cells was found by TADA et al. [29]. In 2016, SABIT et al. [30] showing that the use of procaine combined with carboplatin was the most effective treatment for diminishing the global level of DNA methylation in colon cancer cells. Examined is the central modulation of Procaine acting on the stress axis of limbic system with anti-depressive and psycho-analeptic action [31-36].
Depending from the amount of administered Procaine it is possible to increase the effect to influence pain, inflammation and to reach the other described features of the substance (Figure 1).

Figure 1: Overview of the most important pharmacological and clinical features of Procaine.
Neural therapy is a healing system balancing the Autonomic Nervous System (ANS) to create a state of homeostasis in the nervous system. Physical or emotional trauma, surgery, illness, disease and scars; all have an effect on the ANS and can cause it to become blocked. When it is blocked, a person can be stuck in reactions that feel like fatigues, pain, inflammation, sympathetic dominate patterns (fight and flight stress responses) and the inability to heal. Receiving neural therapy is like hit the reset button on a poorly wired electrical circuitry [37-39]. In neural therapy the local anesthetics procaine are injected into the area of blockage to release or reset the ANS. There is typically quick relief, and the patient can experience a feeling of euphoria and energy, emotional release and release of long-held toxins. This is the body moving from a blocked state to a state of homeostasis. It improves flow between the sympathetic and parasympathetic branches of the nervous system without interference and without side effects [38-40].
The pathology of neurogenic inflammation is well established [41,42]. Ligaments, tendons and joints have TRPV1-sensitive C pain fibre innervations. “When these C pain fibres are irritated anywhere along their length they will transmit ectopic impulses in both forward (pro-dromic) and reverse (anti-dromic) direction [43].”
The forward direction of the nerve signal will cause pain perception as the signal travels through the posterior root ganglia up to the brain. You will also have a local reflex action from the spinal cord ventral horn cells out to the muscle fibers, which will cause a reflex muscle spasm [39,43]. The reverse (anhidrotic) signal will travel to the blood vessels where substance P is released causing swelling and pain. The nerves themselves also have a nerve supply called the Nervi Nervorum (NN) [38,42]. In a pathological state, the NN can release substance P (Sub P) and Calcitonin Gene Related Peptide (CGRP) onto these C pain fibres [43]. Substance P and CGRP are known to cause pain, swelling of the nerve and surrounding tissue [39,47]. Neurogenic inflammation is the physiological process by which mediators are released directly from the cutaneous nerves to initiate an inflammatory reaction. This results in production of local inflammatory responses including erythema, swelling, temperature increase, tenderness, and pain [48-52]. Fine unmyelinated afferent somatic C-fibers, which respond to low intensity mechanical and chemical stimulations, are largely responsible for the release of inflammatory mediators. When stimulated, these nerve fibers in the cutaneous nerves release active neuropeptides – substance P and calcitonin gene related peptide (CGRP) rapidly into the microenvironment, triggering a series of inflammatory responses [37,38,48-52]. Under normal circumstances, peripheral tissue damage in the body will cause sensory neurons to send an impulse via the dorsal root ganglion into the central nervous system (CNS) for further processing. In some cases, instead of the impulse being transmitted centrally, it may shoot down the axon directly, causing neuropeptides release at the distal end of the neurons [38,48]. Consequently, the release of neuropeptides by the irritated neurons induces inflammation in the distal tissues. Sustained inflammation was also suggested to be caused by persistent backfiring [38,48].
Another proposed mechanism is known as neurogenic switching. Under this mechanism, the sensory impulse generated locally gets normally transmitted from the site of activation to the CNS, which then creates an efferent signal to regulate the inflammation. However, the signal is rerouted via the CNS to a distant location and produces Neurogenic inflammation at the second location. In fact, neurogenic switching was further illustrated using the multiple chemical sensitivity syndromes, in which detection of chemical irritants by the respiratory system triggers inflammatory responses in various secondary organ systems. Similarly, this neuronal pathway can be a possible explanation [38,44,45]. Complex regional pain syndrome (CRPS) is a multi-system chronic pain disorder. It is characterized by pain and inflammation with abnormalities in the sensory, trophic, autonomic, and motor systems [46]. Commonly reported in patients after a stroke, surgery, or bone fracture, CRPS is a complication that can possibly cause damage to the peripheral nerves.
In fact, sympathetic innervation becomes evident after the nerve injury caused by CRPS [38,40,46]. CRPS can be further classified into Type I and Type II, with Type I being reported in the majority of patients experiencing CRPS. In Type I CRPS, also known as reflex sympathetic dystrophy, nerve lesions are usually not observable. On the other hand, evidence of nerve damage is generally present in Type II CRPS, making the condition more painful and harder to control [37,40,48]. Clinically, CRPS patients are presented with severe pain, inflammatory symptoms, allodynia, thermal and mechanical hyperalgesia, changes in sweating, abnormal nail and hair growth, and muscle weakness. Patients may also experience paresthesia or sensation loss in affected sites [38,46,49,51].
Since a long-time it is prevalent to finish a neural therapy session with an i.m or i.v shot of 25 till 50 mg Procaine to reach a systemic action. The pure Procaine infusion was firstly described by SEIFEN et al. [36,53] and was mostly used as a continuous treatment in cases of acute pancreatitis [54-56] and for epidural anaesthesia in infants, children and risk patients, which underlines the low toxicity of the substance [57-64]. O´Donnell et al. reported about the use of procaine infusion to block the cardiac nerves [65].
Presently it is reported that long-term relaxing, anti-depressive and anxiolytic effects are often observed when i.v applications or short term infusions of Procaine are given [66,67]. It has been demonstrated that when procaine is administrated intravenously in humans, it increases blood flow of anterior para-limbic zones and the amygdala cerebral [68] as well as to improve hemodynamic effects of the heart [69]. Other areas of limbic system have been studied when procaine is administered in animal models, finding action on many muscarinic cholinergic receptors of hippocampus. Several authors have been reported procaine actions on many biochemical systems such as dopamine, norepinephrine, serotonin, glutamate, among others. For these reasons, procaine is considered as useful for studying limbic system and emotions [70,71].
In a recent research, it has been pointed out that procaine injection into the ventral segmental area is able to suppress temporarily the fear conditioned avoidance response in rats and also acts on hippocampus theta rhythms which are related with arousal and attention [72]. Apparently, the metabolites of Procaine are responsible for the additional pharmacologic actions. DEAE is able to act as an anti-inflammatory due to the inhibition of the fatty acid amide hydrolase which causes an increase in endocannabinoid levels [73,74]. The second metabolite PABA operates as an antihistamine, capillary sealant and as a stabilizer for the membranes due to the ester binding with ceramide [75-77].
With the aim of combining the well-known pure alkaline infusion [78] and the pluripotent features of Procaine, the first study was published as the so-called “neural infusion therapy” in 1997 [79]. After impressive positive results were demonstrated in chronic pain patients [80], the method gained popularity very fast in the German-speaking countries and was incorporated into textbooks of pain and neural therapy [81,82]. Glusa et al. were also able to confirm the vasodilatation effect of the ProcaineBase-mixture by using an animal model [83]. An increase of intra-cellular Procaine concentration due to the addition of sodium bicarbonate [84] and an accelerated initial effect were also observed in animal studies [85,86]. The continuous application of Procaine-Base by the use of a medical pump demonstrated impressive results in many severe cases of pain and inflammation [87-89].
With the osteoarthritis model of rats, the anti-rheumatic and jointprotective action of Procaine- Base after intra-articular injection was clearly superior compared to giving the drug Dexamethason [90].
The primary aim of additionally adding the natural buffer-base sodium bicarbonate was its plasmatic degradation influence on Procaine due to the action of serum esterase. All local anesthetics have the common characteristic of general build-up and ionization. These characteristics are essential for their action on the voltage-dependent sodium channels. The unloaded Procaine molecule represents the transporting structure which is able to permeate.
The loaded form, Procaine-H+ (ionized form) bind the sodium channel receptor and thus block the propagation of an impulse. By changing the pH value of the solution and the terrain, the ionized and non-ionized forms of Procaine can be influenced [91]. It is known that different sodium bicarbonate concentrations can influence the intracellular pH [92].
Initially it was postulated that under more alkaline conditions the conversion of Procaine to para-amino-benzoic acid (PABA) and diethylamino-ethanol (DEAE) will be distinctly reduced. Contrary to this assumption it is believed that after intravenously injecting ProcaineBase it gets diluted in the blood of big vessels leading to a quick drop in pH reaching normal physiological levels. In addition, the pulmonary circulation will cause a respiratory compensation of alkalosis. The actual retardation of Procaine degradation can be explained as follows: The pH-dependent dissociation shift explained above will result in increased amounts of well-penetrating transport forms. This is generally typical for all local anesthetics and thus 3-40% of the liberated base is present depending on the pKa value of the anesthetic drug. Besides the distribution in a steady state, the speed of distribution is also important. The speed of distribution is the limiting factor meaning that the diffusion through the membrane is the speed determining step [93]. The distribution depends directly on the lipophilicity of the agent. By shifting the pH, the lipophilic features are changed. A higher amount of free base implies also a higher amount for permeation, which is immediately available to the surrounding tissue and cannot be metabolized so easily by the serum esterase [55].
If there is no prior information concerning the tolerance of Procaine before the infusion, we recommend making a test application of one drop Procaine 1% into the conjunctiva. Normally, immediately redness (due to increased blood flow) can be observed, a numbness sensation and perhaps a quick burning sensation (due to HCl) can be reported by the patient. If the burning pain persists for some minutes, please abstain from parenteral infusions. It is important to highlight that only Procaine-HCl with a pharmaceutical permission for i.v application and without any preservatives (e.g. parabens) should be used.
We recommend to start with a dosage of 50-100 mg Procaine-HCl and 20 ml sodium hydrogen carbonate (8.4%) diluted in a 250 to 500 ml carrier solution. Meanwhile the isotonic sodium chloride solution, used routinely for many years can be exchanged by a similar electrolyte solution to prevent hypernatremia. The infusion takes place for approximately 45-60 minutes. By adding increments of 50 mg ProcaineHCl and 10 ml sodium bicarbonate (8.4%), the Procaine-Base infusion will be titrated until the desired therapeutic effect has been reached. For a normal-weight person the maximal dosage of Procaine-HCl is 300 mg (Table 1). In patients with cardiovascular risk factors we recommend the use of a surveillance technique (EKG, oximetry) for dosages above 300 mg Procaine-HCl. It is advised to ensure an after- treatment observation period of 30 minutes. Furthermore, it is advised to avoid driving for about one hour after treatment. Because of the stability of the Procaine-Basemixture it should be used up within two hours [because of progressing degradation of Procaine].
| Procaine dosage 1% | Sodium hydrogen carbonate dosage 8.4% | Sodium chloride 0.9% | Total volume |
| 100 mg = 10 ml | 20 ml | 500 ml | 530 ml |
| 200 mg = 20 ml | 40 ml | 500 ml | 560 ml |
| 30 mg = 30 ml | 60 ml | 500 ml | 590 ml |
a. Table of dosage in case of using Procaine 1%
| Procaine dosage 2% | Sodium hydrogen carbonate dosage 8.4 % | Sodium chloride 0.9% | Total volume |
| 100 mg = 5 ml | 20 ml | 500 ml | 525 ml |
| 200 mg = 10 ml | 40 ml | 500 ml | 550 ml |
| 300 mg = 15 ml | 60 ml | 500 ml | 575 ml |
b. Table of dosage in case of using Procaine 2%
Table: 1
Without any prior acid-base diagnostic the Procaine-Base infusion should not be administered more than three times per week with a minimum of one-day break between treatment days. A series of 6 to 10 infusions depending on the medical condition have been approved.
The classic blood parameters for inflammation like blood sedimentation rate and C-reactive protein (CRP) should improve after a series of Procaine-Base infusions. Almost always after four till six applications the patients report much better mood and improved overall condition. If there is a positive reaction to the treatment (so-called “responders”, in approx. 80 % of patients) it is advised especially in chronic diseases, to continue with a long-term therapy using the helpful dosage for longer intervals, e.g. one till twice a month [34,35,94].
The hypersensitivity to Procaine (also called “para-group allergy”) reported in old textbooks with an increased allergy rate has not been confirmed yet [95,96]. A published meta-analysis monitoring vital data during Procaine-Base infusions in 5.698 patients, which included a complete documentation of blood pressure, oximetry and pulse rate before, during and after the infusion, showed no statistically significant differences between the main groups [97]. The mean values, standard deviations and ranges of assessed vital parameters are shown in (Table 2). Even in high-dosed ranges of Procaine, the stability of values was remarkably high. Most treatment ranges registered used medium concentrations of Procaine (100-300mg) and sodium hydrogen carbonate (8, 4%, 20-60 ml) which are considered as standard daily practice. Just a slight tendency of increased average blood pressure was found with a high dosage of Procaine (over 500mg) and especially sodium bicarbonate (8, 4%, over 100ml).
Furthermore, it is of interest that the measured blood pressure had no significant difference after 15 and 30 minutes of the start of the infusion (Table 2).
| Procaine-HCl+ Na-HCO3 8,4 % | 100mg + 20ml | 200mg + 40ml | 300mg + 60ml | 400mg + 80ml | 500mg + 100ml | over 500mg + 100ml |
| analyzed data (n) | 215 | 2241 | 3031 | 88 | 105 | 18 |
| PULS rate per | 74,8 ± | 74,2 ± | 74,5 ± | 74,4 ± | 75,8 ± | 73,1 ± |
| Minute (Min.) | 13,1 | 12,4 | 12,0 | 13,1 | 11,7 | 12,6 |
| after 15 Min. | [44 - 121] | [55 - 145] | [48 - 164] | [48 - 123] | [59 - 105] | [55 - 95] |
| 02-saturation (%) | 95,3 ± 4,6 | 95,4 ± 4,9 | 95,7 ± 5,9 | 95,1 ± 4,5 | 95,3 ± 5,8 | 95,3 ± 2,2 |
| after 15 Min. | [80 - 100] | [79 - 100] | [77 - 100] | [81 - 100] | [88 - 99] | [85 - 98] |
| RR systolic | 145,6 ± | 145,3 ± | 145,7 ± | 144,6 ± | 139,2 ± | 151,0 ± |
| after 15 Min. | 21,5 | 21,0 | 21,8 | 20,5 | 24,1 | 14,1 |
| [90 - 220] | [84 - 214] | [74 - 221] | [89 - 198] | [83 - 210] | [115 - 190] | |
| RR diastolic | 83,0 ± | 83,9 ± | 84,5 ± | 86,4 ± | 81,7 ± | 87,6 ± |
| after 15 Min. | 10,9 | 11,5 | 11,1 | 12,0 | 15,3 | 13,0 |
| [45 - 146] | [47 - 137] | [44 - 135] | [54 - 133] | [53 - 116] | [70 - 107] | |
| RR systolic | 139,8 ± | 140,1 ± | 139,2 ± | 134,5 ± | 127,0 ± | 140,6 ± |
| after 30 Min. | 19,7 | 19,2 | 19,1 | 22,2 | 22,1 | 19,1 |
| [68 - 210] | [80 - 205] | [80 - 210] | [97 - 206] | [86 - 208] | [105 -198] | |
| RR diastolic | 80,4 ± | 80,7 ± | 80,9 ± | 81,1 ± | 81,7 ± | 82,9 ± |
| after 30 Min. | 11,9 | 11,2 | 11,9 | 12,8 | 12,1 | 11,8 |
| [48-122] | [47-140] | [44-155] | [45-122] | [55-127] | [76-116] |
Table 2: Mean [M], simple standard deviation [± SD], Minimum and Maximum [from to] protocolled surveillance data during infusion with different dosages of Procaine and sodium bicarbonate in isotonic sodium chloride basic solution [n=5698], taken from Oettmeier and Reuter, 2000 [63].
After over 500.000 applications of procaine infusions according to the described regime in our hospital and outpatient department, we have not observed one case with long-term or severe side effects. No case was registered with a serious allergic emergency situation, which underlines the observations of Becke concerning the huge therapeutic safety of Procaine [73]. Sometimes patients report of heart palpitation (6%) and profuse sweating (5%). According to our experience patients who are using nitro compounds, calcium antagonists and beta- blockers seem to have a higher disposition to these side effects. Apparently in such cases the reflective and over-segmental disinheriting effect of the anesthesia dominates over the negative-ionotropic and negative-rhythm tropic potential of Procaine. In approximately 6% of patients a short-time reduction in blood-pressure and vasovagal syncope situations can occur during the application. All these symptoms disappear within few minutes, especially after reducing the infusion speed [34,78].
Some patients report about sleep disorders (5%) and a general hyperactive feeling till one day after finishing the infusion, which does not reduce the physical working capacity. Approximately 4, 5% of treated persons complained of temporary headaches and slight vertigo. Especially during the first couple of infusions such reactions can occur in scope of a so-called “first reaction” (HERING´s effect) according to the holistic thinking in neural therapy [1] and homeopathy [98].
The multiple therapeutic effects of Procaine in combination with an alkaline additive are responsible for the enormous palette of medical indications (Table 3). Especially all kind of pain, inflammatory and autoimmune diseases, vegetative imbalances in addition to the complementary cancer treatments are of primary importance.
It is important to emphasize that according to the above described procedure of Procaine-Base- infusion, a daily application of such a high dose of sodium bicarbonate is inacceptable.
Patients having a metabolic alkalosis with a reduced compensatory ability in acid-base balance are increasing. This is found in cases of overpotentiating, advanced stages of cancer, liver weakness and putrefaction dysbiosis of the large intestine. Furthermore, the use of antacids, alkaline powders, loop diuretics and too much sodium intake enhances the shift in the acid- base-balance towards alkalosis [99,100]. For the practical analysis of the acid-base balance we prefer the venous blood titration system Buffy® over the arterial blood gas analysis (aBGA) [101-104] because it is haematocrit-adapted and calibrated to 37°Celsius. The test gives very good information about the buffer capacity of whole blood and plasma and indicates exactly the amount of base needed [105,106]. Metabolic alkalosis can also occur in hyponatremia, hypokalaemia and in increased ammonia levels (in EDTA plasma).
In cases of inflammatory, cardiac and renal dysfunctions, rheumatic and pain-related diseases a metabolic acidosis is mostly likely detected [107]. These patients have an increased need of a buffer base and should receive sodium bicarbonate ranging from 60-120 ml (8, 4% solution) in addition to Procaine.
In contrast to cases of metabolic alkalosis there is only a small or no need of additional base treatment. In this case, we only administer infusions of Procaine-HCL together with a carrier solution.
After the empirical start twenty years ago the Procaine-Base-infusion treatment meanwhile reached a well-accepted level of clinical importance and has advanced as routine therapy in many hospitals and outpatient departments for pain treatment, rehabilitation and natural medicine. Even if scientific findings convincingly confirm the Procaine-Base mechanism of action, spectrum of indications and the individualized application, more research and scientific studies are warranted. The treatment is safe in the right practical application.
It is very important for the authors of this article to emphasize that the treatment method of Procaine is safe, conducted properly, especially to individualize the therapy according to the acid-base-homeostasis and clinical parameters of the patient [91]. Finally, it is noticeable that the method is an addition, not a replacement for neural therapy injections, especially for treatment of neuro-modulative triggers and nerve blocks.
Download Provisional PDF Here
Article Type: Review Article
Citation: Reuter URM, Oettmeier R and Nazlikul H (2017) Procaine and Procaine-Base-Infusion: A Review of the Safety and Fields of Application after Twenty Years of Use. Clin Res Open Access 4(1): doi http://dx.doi.org/10.16966/2469-6714.127
Copyright: © 2017 Reuter URM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access