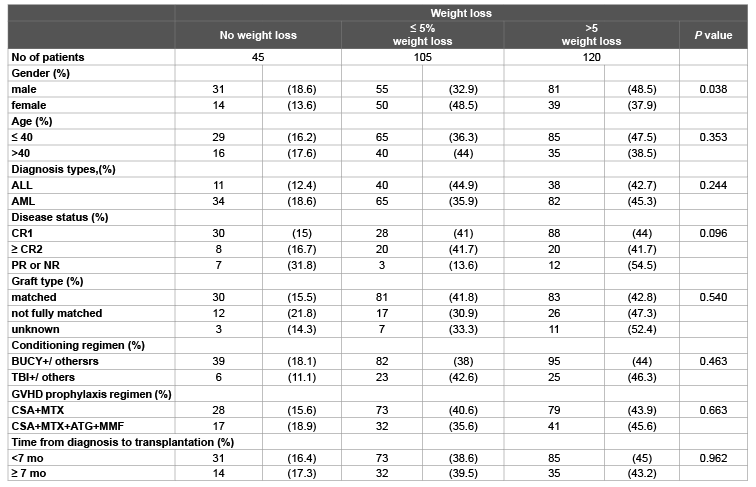
Table 1: Baseline characteristics by weight change groups in allo-HSCT patients (n=270)
Jing Yang1,2# Sheng-Li Xue3# Xiang Zhang3# Li-Qiang Qin1 Yue-Ping Shen4* De-Pei Wu3*
1Department of Clinical Nutrition, The First Affiliated Hospital of Soochow, University, China*Corresponding author: Yue-Ping Shen, Department of Biostatistics and Epidemiology, School of Public Health, Soochow, University, 199 Renai Road, DushuLake Higher EducationTown, Suzhou, 215123 China, Tel: +8613962148023; Fax: +086-512-65883323; E-mail: shenyueping@suda.edu.cn
De-Pei Wu, Department of Hematology, The First Affiliated Hospital of Soochow University, 188 Shizi Street, Suzhou, 215006 China, Tel: +8615370002998; Fax: +086-512-65228072; E-mail: wudepeinutrition@163.com
The present work was performed to investigate the association between peri-transplant weight loss and the overall survival (OS) of patients with allogeneic hematopoietic stem cell transplantation (allo-HSCT). Data from 310 adults who were diagnosed with acute leukemia and underwent allo-HSCT between March 2001 and December 2011 were analyzed. Demographic and clinical data were collected from medical records. Weight loss was categorized into to “no weight loss”, 0- ≤ 5%weight loss and >5% weight loss, respectively. Cox proportional hazards models was used to estimate hazard ratios with 95% confidence intervals. The median follow-up time among the patients was 19.5 months (interquartile range=7.6 to 37.7). A total of 93 (34.4%) people died within the follow-up period. For the overall comparison of probabilities of OS, there were no statistically significant difference among the weight change groups (P=0.732), corresponding OS probabilities at 3 years were 70.3% [56.5%-84.1%], 76.2% [67.4%-85%], and 76.5% [68.5%-84.5%], respectively. After adjustment the potential confounders, patients who had weight loss ≤ 5% and >5%weight loss did not have poor OS compared with patients who had no weight loss (HR=1.31, 95% CI: 0.70–2.45, HR=0.96, p=0.394; 95% CI: 0.52-1.79, p=0.896). We did not find an association between peri-transplant weight loss and OS in allo-HSCT patients. Further study will be needed to explore the effects of weight loss on allo-transplant patients. Demographic and clinical data were collected from medical records. Weight loss was categorized into to “no weight loss”, 0- ≤ 5%weight loss and >5%weight loss, respectively. Cox proportional hazards models was used to estimate hazard ratios with 95% confidence intervals. The median follow-up time among the patients was 19.5 months (interquartile range=7.6 to 37.7). A total of 93 (34.4%) people died within the follow-up period. For the overall comparison of probabilities of OS, there were no statistically significant difference among the weight change groups (P=0.732), corresponding OS probabilities at 3 years were 70.3% [56.5%- 84.1%], 76.2% [67.4%-85%], and 76.5% [68.5%-84.5%], respectively. After adjustment the potential confounders, patients who had weight loss ≤ 5% and >5%weight loss did not have poor OS compared with patients who had no weight loss (HR=1.31, 95% CI: 0.70–2.45, HR=0.96, p=0.394; 95% CI: 0.52-1.79, p=0.896). We did not find an association between peri-transplant weight loss and OS in allo-HSCT patients. Further study will be needed to explore the effects of weight loss on allo-transplant patients.
Weight loss; Overall survival; Allogeneic hematopoietic stem cell transplantation; Nutrition; Outcome
Allogeneic hematopoietic stem cell transplantation (HSCT) is an wellestablished treatment for a variety of hematologic malignancies [1]. With the advancement in transplantation techniques and supportive care, the risk of acute complications has been significantly reduced among transplant patients, and early treatment-related mortality also decreased. In turn, much of the research focus has shifted to the long-term survival of allo-HSCT. Malnutrition after transplantation is a restraining factor of long-term survival.
Weight loss, a commonly utilized measure for indicating nutrition status in adults, is frequently observed in cancer patients [2-4]. A weight loss of greater than 10% of the pre-illness body weight may occur in up to 45% of cancer patients [5]. It is reported that a critical weight loss place cancer patients at nutrition risk and is associated with low quality of life, poor response to chemotherapy, reduced performance status and shorter survival than in non-weight losing patients [6-8].
Weight loss is also frequently observed after allogeneic hematopoietic stem cell transplantation, especially during peri-transplant period [9]. Identification of nutritional high- risk patients may provide further clues in developing clinical intervention to improve survival [10]. However, the impact of peri-transplant body weight loss on HSCT prognosis has not been addressed. Is peri-transplant weight loss also an unfavorable prognostic factor for overall survival of allo-HSCT? Analysis the association between peri-transplant weight loss and overall survival in HSCT patients may be required. Therefore, in this study, we investigated the prognostic influence of peri-transplant weight loss.
This was a single-center retrospective cohort study which included adult patients (age18 ≥ years) who were diagnosed with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML), receiving Busulfan and cyclophosphamide (BUCY) or total body irradiation (TBI) based conditioning regimen. The transplantation was operated in the First Affiliated Hospital of Soochow University from March 2001 until December 2011. Peri-transplant weight loss was calculated as weight at the time leaving laminar flow room minus weight at the time before receiving conditioning regimen. A number of studies suggested that a greater than 5% body weight loss during the course of cancer indicated malnutrition and is clinical meaningful, Weight loss was categorized into to “no weight loss”, 0- ≤ 5%weight loss and >5% weight loss.
The end point was OS, which was defined as the time from date of transplantation to date of death. The other variables included age, gender, diagnosis, disease status, graft types, conditioning regimen, GVHD prophylaxis regimen, and time from diagnosis to transplantation. All of the demographic and clinical data were collected by reviewing the medical records. The last follow-up time was May 13, 2014. Our methods were carried out in accordance with the approved guidelines. All the experimental protocols were approved by the ethic committee of the first affiliated hospital of Soochow university.
Patient-, disease-, and transplant-related characteristics were compared among weight loss by using Chi-square test. Univariate probabilities of OS were estimated by using the Kaplan-Meier method. Log-rank test was used for comparing survival curves. Cox proportional hazards regression model was used to estimate the association between the weight loss groups and outcomes of death after adjusting for the potential confounders: age (≤ 40=1; >40=2), gender (male=1; female=2), diagnosis(ALL=1; AML=2), disease status before transplantation (CR1=1; ≥ CR2=2; PR or NR=3), graft types (matched=1; not fully matched=2; unknown=3), conditioning regimen (BUCY+/others=1; TBI+/others=2), GVHD prophylaxis regimen (CSA+MTX=1; CSA+MTX+ATG+MMF=2), time from diagnosis to transplantation (<7mo=1; ≥ 7mo=2).
There were 310 patients who met the eligibility criteria. The variables most often missing was survival time (n=19), followed by weight at the time leaving laminar flow room or weight at baseline (n=12), then diagnosis time (n=4) and graft versus host disease (GVHD) prophylaxis regimen (n=5). After excluding the missing data, the final sample size for analysis was 270.
The comparisons for patients-, disease-, and transplant-related characteristics among the weight change groups are presented in Table 1. One hundred and sixty seven (61.9%) patients in our study were male and 179 (66.3%) patients were younger than or equal to 40 years. The number of the patients with no weight loss, ≤ 5% weight loss and >5% weight loss was 45 (16.7%), 105 (38.9%) and 120 (44.4%), respectively. Except gender, there were no significantly different among weight loss groups in these variables including age, diagnosis, and disease status, graft type conditioning regimen, GVHD prophylaxis regimen and time from diagnosis to transplantation.
Median follow-up time among patients were 19.5 months [IQR=7.6- 37.7], during follow-up, 34.4% patients died (n=93). For the overall comparison of probabilities of OS, there were no statistically significant difference among the weight change groups (P=0.732), corresponding OS probabilities at 3 years were70.3% [56.5%-84.1%], 76.2% [67.4%-85%], and 76.5% [68.5%-84.5%], respectively.
The multivariate analysis of hazard ratio(HR) for overall survival adjusted for age, gender, diagnosis, disease status, graft type, conditioning regimen, GVHD prophylaxis regimen, time from diagnosis to transplantation. After adjustment, patients who had weight loss ≤ 5% and >5% weight loss did not have poor OS compared with patients who had no weight loss (HR= 1.31, 95% CI: 0.70–2.45, HR=0.96, p=0.394; 95% CI: 0.52-1.79, p=0.896).
Involuntary peri-transplant weight loss is a major problem in patients with allo-HSCT. A number of factors are associated with the development of weight loss which include the systemic effects of the disease and side-effects of condition regime treatments (e.g. mucositis, taste alterations, or nausea), graft versus host disease (e.g. diarrhea, abdominal pain, gastrointestinal bleeding), and psychological factors (e.g. depression) [11-13].
In our study, the proportion of patients having weight loss ≤ 5% was 38.9%, and the proportion of patients with >5% weight loss was 44.4%. However, neither of patients with ≤ 5% weight loss and >5% weight loss had poor prognosis compared with patients with no weight loss. The lack of an association may be caused by the following reasons. The first reason possibly relate to the confounding effect of edema and as cites on body weight measurement [14], severe malnutrition and hypoproteinemia can lead to fluid retention, weight loss can be disguised by an increase in fluid retention. Body weight remained constant or even increased [15,16]. Therefore, the real association between weight loss and prognosis can been influenced. In addition, we only studied the peri-transplant weight change and did not take into account the weight loss in the later period of post-transplant, in fact, chronic GVHD and long-term steroid treatment are also associated with a decline in lean body mass and weight loss [17]. According to Lenssen et al. [12] study, the incidence of weight loss was 33% in patients with extensive cGVHD and 19% in patients with limited cGVHD. Weight loss should be recorded throughout the whole period after transplantation. The possibility of selection bias and survival bias also could not be ruled out as only patients treated in our hospital were included. Besides, approximately 10% of the patients had missing data. The patients had higher mortality and therefore their exclusion from the analysis might have produced bias results.
Although patients with >5% weight loss did not have poor overall survival than patients with no weight loss in our study. Appropriate nutritional assessment, nutritional counseling and nutritional intervention may still need [11,18]. Especially for underweight patients, who were more susceptible to severe side effects, such as mucositis, dysphagia and more likely to be malnutrition. Weight loss and prognosis of allotransplant patients deserve further investigation. Except for minimizing the limitations mentioned above, we should take into consideration body composition (fat mass and fat-free mass), the distribution of adiposity and other index including reduced food intake and systematic inflammation in the following study.

Table 1: Baseline characteristics by weight change groups in allo-HSCT patients (n=270)
We did not find an association between peri-transplant weight loss and overall survival in allogeneic hematopoietic stem cell transplant. However, in view of the limitation of our study and the harmful effects of malnutrition, we recommend further study will be needed to explore the effects of weight loss on allo-transplant patients.
We would like to thank members of Department of Hematology for excellent technical assistance.
All authors declare that they have no competing financial interests.
Download Provisional PDF Here
Article Type: Research Article
Citation: Yang J, Xue S-L, Zhang X, Qin L-Q, Shen Y-P, et al. (2016) Impact of Peri-Transplant Weight Loss on Overall Survival in Allogeneic Hematopoietic Stem Cell Transplant. Clin Res Open Access 2(2): doi http://dx.doi.org/10.16966/2469-6714.114
Copyright: © 2016 Yang J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access