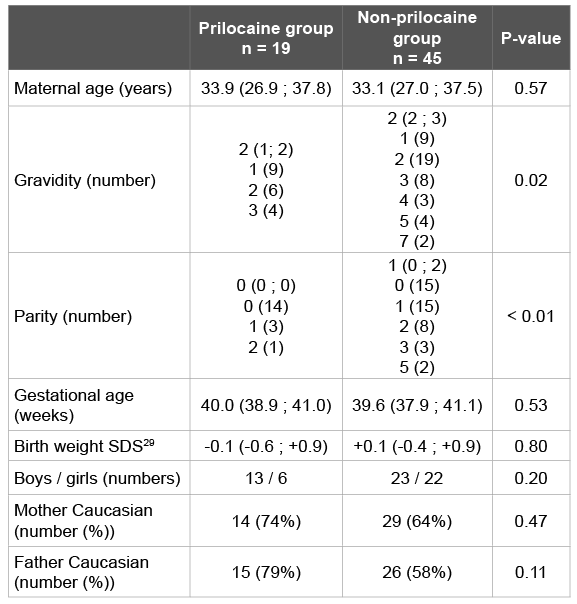
Table 1: Baseline characteristics
Muiño-Mosquera L1 Lem AJ2 Thio GKKE3 Aleman J1 Franssen EJF3 Valerio PG1 van Kempen AAMW*1
1Department of Paediatrics, University Hospital Ghent, De Pintelaan 185, 9000 Gent, Belgium*Corresponding author: van Kempen AA, Department of Paediatrics and Neonatology, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands, Tel: +31 20 599 3528; E-mail: a.vankempen@olvg.nl
Background: Prilocaine, a local anaesthetic used in obstetrics for episiotomy, has been associated with methaemoglobinaemia in newborns. The aim of this study was to evaluate the occurrence of clinical and subclinical methaemoglobinaemia in prilocaine-exposed and non-exposed newborns.
Methods: In a prospective pilot study methaemoglobin levels were measured in a group of 64 newborns; 19 exposed and 45 not exposed to 10ml prilocaine 1%for maternal episiotomy. Additionally, in a retrospective study the occurrence of methaemoglobinaemia in prilocaine-exposed and non-exposed newborns was compared in a 1-year cohort (n=2223) of infants in whom blood gas analysis was performed for clinical purposes (n=358).
Results: None of the newborns in the prospective study had clinical or subclinical methaemoglobinaemia. Methaemoglobin levels were low and similar in prilocaine-exposed and non-exposed newborns (median of 0.3% and 0.4% respectively in umbilical cord blood and 0.2% in both groups in the newborn 2-3 hours after birth). In the retrospective cohort, 2/541 prilocaine-exposed newborns showed clinical methaemoglobinaemia (0.37%). Both responded well to methylene blue. Similar to in the prospective study, there were no newborns with subclinical methaemoglobinaemia, and the methaemoglobin levels were similar in prilocaine-exposed and non-exposed newborns.
Conclusions: The estimated incidence of neonatal methaemoglobinaemia after prilocaine for episiotomy is 0.37%. Subclinical methaemoglobinaemia did not occur, implicating that symptom-free newborns do not need routine methaemoglobin measurements.
Anaesthesia; Obstetrical; Infant; Newborn; Maternal-fetal exchange/Drug effects; Methaemoglobinaemia; Prilocaine
Prilocaine hydrochloride (Citanest® ) is a local anaesthetic of the amide type. Other anaesthetics such as bupivacaine, mepivacaine and lidocaine belong to the same group. In obstetrics, local anaesthetics are used for episiotomy. Compared to other local anaesthetics, prilocaine has the advantage to cause less maternal cardiovascular and neurological sideeffects [1,2]. However, it readily passes the placenta and may induce methaemoglobinaemia in the newborn [3,4].
Methaemoglobin is an altered state of haemoglobin in which ferrous (Fe2+) irons of haeme are oxidized to the ferric (Fe3+) states. The oxygen dissociation curve is shifted leftwards in methaemoglobinaemia, as methaemoglobin binds oxygen more easily, but unloads it more reluctantly. This results in decreased oxygenation of the tissues and therefore clinical cyanosis.
Neonatal methaemoglobinaemia resulting from prilocaine administration to the mother was first described in 1972 [5,6]. Since then, several case reports describing this phenomenon were published [7-12]. Different dosages of prilocaine, ranging from 10ml of prilocaine 1% to 20ml prilocaine 2%, were used in these case reports. In addition, methaemoglobinaemia has been described in children who underwent dental procedures with prilocaine 3% as a topical anaesthetic [13-15]. It has also been described in term and preterm newborns who received an Eutectic Mixture of Local Anesthetics (EMLA® ) cream, containing 2,5% prilocaine and 2,5% lidocaine, in procedures such as circumcision and lumbar puncture [16-19].
Newborns are at higher risk of developing methaemoglobinaemia because of their lower levels of methaemoglobin-reductase, the enzyme responsible for reducing oxidized methaemoglobin, and because of their higher level of foetalhaemoglobin (HbF) which is more easily oxidized to methaemoglobin [20]. Clinical circumstances that cause foetal acidosis, such as foetal asphyxia, placental abruption, and intrauterine infection can enhance the passage of prilocaine across the placenta [3,4].
Because of the risk of neonatal methaemoglobinaemia, the use of prilocaine in obstetrics has been discouraged. In the last seven years five newborns in our hospital developed clinical methaemoglobinaemia after prilocaine administration to the mother for episiotomy. So far, there are no studies estimating the incidence of clinical and subclinical methaemoglobinaemia in newborn infants. Also, there are no studies comparing neonatal methaemoglobin levels in prilocaine-exposed versus non-exposed newborns.
The primary objective of this study was to compare the methaemoglobin levels and the occurrence of clinical and subclinical methaemoglobinaemia in prilocaine-exposed and non-exposed newborns. The secondary objective was to determine whether there was a correlation between serum prilocaine concentrations and methaemoglobinaemia.
The study was conducted at the departments of Obstetrics, Neonatology and Clinical Pharmacy of the Onze Lieve Vrouwe Gasthuis (OLVG), a general and teaching hospital in Amsterdam, the Netherlands. In our hospital 10ml of prilocaine 1% is used as a standard anaesthetic for episiotomy because it has less cardiovascular and neurological side-effects for the mother than other local anaesthetics. The study was approved by the local Medical Ethics Committee. The study was carried out in accordance with the World Medical Association Declaration of Helsinki regarding ethical conduct of research involving human subjects.
All women giving birth at the department of Obstetrics between January and September 2009 were eligible for the study. Exclusion criteria were: hereditary methaemoglobinaemia [21], G6PD deficiency [22], haemoglobinopathy, such as sickle cell disease or thalassaemia [23], the use of other drugs during labour that could cause methaemoglobinaemia, and caesarean section. Women who wanted to donate umbilical cord blood were included in the study. Donation of cord blood was given priority over blood sampling for the study. Written informed consent was obtained from all women included in the study.
The study design was a blinded, controlled, prospective, observational cohort study. A formal power calculation was not possible due to a lack of data on methaemoglobin levels in prilocaine-exposed and non-exposed newborns. Therefore the study was conducted as a pilot study.
The performance of an episiotomy and thus the administration of prilocaine (infiltration with 10 ml of prilocaine 1%) were at the discretion of the attending obstetrician. Women undergoing an episiotomy were included in the prilocaine group. Women not undergoing an episiotomy did not received any neither drug nor placebo and were eligible for the non-prilocaine group.
In the prilocaine group prilocaine concentrations were determined in maternal blood immediately after delivery, in umbilical cord blood and in capillary blood from the newborn two to three hours after birth. Methaemoglobin percentages and blood gas analysis were determined in umbilical cord blood and in capillary blood from the newborn two to three hours after birth. A full physical examination, including heart rate, respiratory rate, transcutaneous oxygen saturation and core temperature, was performed immediately after birth and repeated after two to three hours. The same data were collected in the non-prilocaine group except for the prilocaine concentrations. Blood samples and clinical data from the newborns were collected by a blinded researcher, who was not aware whether the mother received prilocaine or not.
Methaemoglobinaemia was defined as a methaemoglobin percentage above 3% [21]. Subclinical methaemoglobinaemia was defined as methaemoglobinaemia without clinical symptoms like cyanosis or respiratory distress. Prilocaine concentrations >1 mg/l were considered clinically relevant [24].
Serum prilocaine concentrations in the mother, umbilical cord and newborn were determined using liquid chromatography combined with mass spectrometry (LC-MS, 1100 series MSD, Agilent Technologies, Minnesota, USA). Calibration curves were constructed by adding known amounts of prilocaine to serum samples. The limit of quantification was 0.05 mg/l. The biochemical analysis of the blood samples was performed in a co-oximeter (Rapidlab 845, Siemens, Holliston, MA, USA). Methaemoglobin percentage was measured by means of a spectrophotometrical analysis.
Statistical analysis was performed using SPSS Statistics version 21 (SPSS Inc., Chicago, IL, USA). Normally distributed data were analysed using the independent-samples t-test. Not normally distributed data were analysed using the Wilcoxon-Mann-Whitney test. The chi-square test was used for categorical variables. Correlations were analysed using Spearman’s rank correlation.
Normally distributed data are given as mean and standard deviation (SD) while not normally distributed data are given as median and interquartile range.
We counselled 349 women for the study; 117 women agreed to participate (all singleton pregnancies). Sixty four (54.7%) completed the study. Reasons for dropping out were newborn not available for clinical examination (n=26), secondary caesarean section (n=9), failure to obtain any blood sample (n=9, partly due to donation of umbilical cord blood) or withdrawal during the study (n=9).
Baseline characteristics of the prilocaine group (n=19) and the nonprilocaine group (n=45) are shown in (Table 1). Except for gravidity and parity, there were no statistically significant differences between both groups.
Table 2 shows the biochemical data from maternal, umbilical cord and capillary blood of the newborn. There were no cases of methaemoglobinaemia. In fact, methaemoglobin percentages in umbilical cord blood were low in all newborns, with a maximum of 0.8% in the prilocaine group and 1.0% in the non-prilocaine group.
Placental passage of prilocaine was shown by the presence of prilocaine in umbilical cord blood. Prilocaine concentrations in maternal blood were low (maximum 2.13 mg/l). In umbilical cord blood the maximum concentration was 0.62 mg/l. There was no significant correlation between the levels of prilocaine in maternal blood and umbilical cord blood (R² 0,115; p= 0,751).Capillary blood samples of twelve newborns were available for analysis of the prilocaine concentration. Of those samples only two were above the limit of quantification of 0.05 mg/l (0.06 and 0.11 mg/l respectively), but well below the clinically relevant level of 1 mg/l. There was no correlation between the maternal prilocaine concentrations in serum and methaemoglobin percentages in umbilical cord blood (R2 0.078; p=0.43) or in the newborns two to three hours after birth.
Blood gas analysis showed a significantly lower pH in umbilical cord blood in the prilocaine-group. However there was no significant correlation between pH in umbilical cord blood and percentage of methaemoglobin (R² -0,165; p=0,279).
Clinical outcomes were not different between prilocaine and nonprilocaine exposed newborns (Table 3). There were no newborns presenting with central cyanosis.
In our prospective, controlled pilot study we measured methaemoglobin percentages in a group of 64 newborns; 19 exposed and 45 not exposed to prilocaine during labour. None of the newborns developed clinical or subclinical methaemoglobinaemia. Moreover, methaemoglobin percentages in prilocaine-exposed newborns were low, and similar to non-exposed newborns, both in umbilical cord blood and two to three hours after birth. Prilocaine concentrations in maternal, umbilical cord and neonatal blood were low. There was no correlation between the prilocaine concentration and methaemoglobin percentage.
To our knowledge only one (uncontrolled) study was published in which methaemoglobin percentage was measured in 17 prilocaineexposed newborns [25,26]. The highest methaemoglobin percentages were seen two hours after birth, and were higher than in our population (mean 1.8%; maximum 3.7%) but far below the clinical significant level. This could be due to the higher dose of prilocaine used (20 ml prilocaine 1%). A control group was lacking in this study.
The absence of a statistical significant difference in our pilot study could be explained by the small number of patients. However, the medians of the methaemoglobin percentages in the exposed and non-exposed group were quite similar with narrow interquartile ranges, which make it more plausible that a significant difference is truly absent.
A logical consequence would be to continue the inclusion. However, despite much effort, the inclusion rate was very slow. Taking the preliminary results of the prospective pilot study into account, we decided to conduct an additional retrospective study in a year cohort of 2223 infants born in our hospital. Prilocaine was administered to 541 mothers (24.3%). Two newborns in the prilocaine group and one newborn in the non-prilocaine group showed severe clinical methaemoglobinaemia. From this year cohort, the estimated incidence of prilocaine-induced methaemoglobinaemia is 0.37% (2/541 exposed newborns).

Table 1: Baseline characteristics
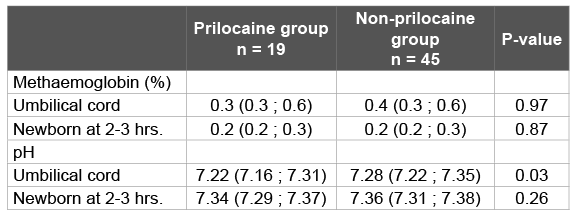
Table 2: Prilocaine concentration, methaemoglobin percentage and pH in maternal, umbilical cord and neonatal blood samples
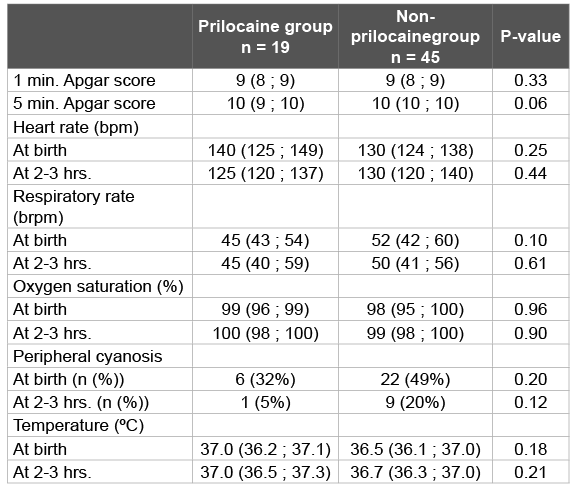
Table 3: Clinical data at birth and at 2-3 hours postnatal age
Bpm: beats per minute; brpm: breaths rate per minute
In 358 (16.1%) newborns without clinical methaemoglobinaemia a blood gas analysis in umbilical cord blood and/or capillary blood was performed for clinical reasons in the first twelve hours after birth. The methaemoglobin percentage was comparable in the prilocaine group (n=136) and the non-prilocaine group (n=222) (Table 4). Maximum methaemoglobin percentage in venous umbilical cord blood was 1.8%, both in the exposed and non-exposed group. In the first six hours after birth the maximum methaemoglobin percentage was 0.8% in the prilocaine group and 1.2% in the non-prilocaine group. These results are in line with the results from the prospective study. More detailed information about the retrospective cohort can be found in the Appendix.
In our pilot study randomization was impossible because the decision to place an episiotomy or not is based on the clinical circumstances during labour and the risks for the mother and the child. Despite this, baseline characteristics did not differ significantly between the groups, except for gravidity and parity. Gravidity and parity were higher in then on-prilocaine group. This finding was expected because episiotomies are more frequently performed in first pregnancies [27]. These baseline variables showed no correlation with methaemoglobin percentage and have no biologically plausible influence on the level of methaemoglobin percentage in the newborn. Therefore, it is unlikely that this difference has affected the results and conclusions of our study.
Clinical outcomes such as central cyanosis, heart rate or respiratory rate, oxygen saturation and Apgar score were not different between the groups in the pilot study. The pH in umbilical cord blood was lower in the prilocaine group. This result is probably directly related to the fact that episiotomies are performed to shorten labour in case of foetal distress. Fetal acidosis is a known risk factor for developing methaemoglobinaemia [3,4]. We found no correlation between the pH in umbilical cord blood and the percentage of methaemoglobin in our study.
Passage of prilocaine through the placenta was shown by the presence of prilocaine in umbilical cord blood. However, all prilocaine concentrations in umbilical cord and capillary blood samples were <1 mg/l, which is considered clinically irrelevant [24]. In the study of Biscoping et al. the mean prilocaine concentration after 20 ml prilocaine 1% (twice the dose used in our study) was higher than in our study, but still below the concentration considered clinically relevant: 0.57 mg/l and 0.29 mg/l in mother and newborn respectively [25,26].
In our study the fetal/maternal prilocaine total concentration was approximately 0.5 (0.2/0.37; Table 2). This contrasts with earlier reported ratios of 1 [4]. This finding suggests that the transfer of prilocaine across the placenta is not complete or in equilibrium in our study. An explanation could be a short interval from episiotomy to delivery. It should be noted that, since prilocaine is a weak base (pKa = 7.89) and the fetal acidosis increases the maternal/fetal pH gradient, an accumulation of free drug in the fetus and possible side effects such as methaemoglobinaemia can still not be ruled out. Fortunately, clinical relevant methaemoglobinaemia was not observed in the newborns in the present study population.
Prilocaine is metabolized into metabolites, amongst others o-toluidine, by enzymes that may cause methaemoglobinaemia [1,2]. Recently, it has been shown that carboxylesterases and cytochromes P450 are involved in prilocaine-induced methaemoglobinaemia [28]. Since these enzymes have variable expressions in both mothers and newborns, one may expect variable formation of metabolites (o-toluidine and hydroxylated o-toluidine) and consequently variable levels of methaemoglobin. However, the fact that no clinical and biochemical problems were observed in the exposed newborns, is reassuring.
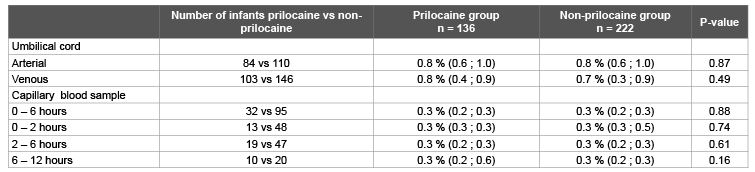
Table 4: Methaemoglobin percentages in the retrospective cohort
In choosing a local anaesthetic, the risk of neonatal methaemoglobinaemia must be weighed against other side-effects in the mother and the newborn. Publications in the literature are mostly casereports; with so far only one uncontrolled study. In our study we found no statistically significant differences in methaemoglobin percentages between prilocaine-exposed and non-exposed newborns. The estimated incidence of prilocaine-induced methaemoglobinaemia is 0.37%. Therefore, we conclude that methaemoglobinaemia is an uncommon complication of prilocaine administration for episiotomy. Subclinical methaemoglobinaemia did not occur; hence this seems to be an on/off phenomenon.
In all cases of methaemoglobinaemia that occurred in our hospital, evident clinical symptoms appeared within the first three hours after birth. All cases responded rapidly to intravenous administration of methylene blue (1 mg/kg bodyweight).
In choosing prilocaine as a local anaesthetic for episiotomy, one should be aware of the possibility of methaemoglobinaemiain the newborn. Observation of the newborn for at least three hours after birth is therefore advised. Routine measurement of the methaemoglobin percentage is not mandatory. Cyanosis in a newborn exposed to prilocaine should raise the suspicion of methaemoglobinaemia and, if confirmed, it should be treated with methylene blue.
The authors thank Mrs. L.M. Dijksman, statistician, Research Bureau, Onze Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands, for help with the statistical analysis.
The study was not funded.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Muiño-Mosquera L, Lem AJ, Thio GKKE, Aleman J, Franssen EJF, et al. (2015) Neonatal Methaemoglobin Levels after Prilocaine Exposure for Maternal Episiotomy. Clin Res Open Access 1(2): doi http://dx.doi.org/10.16966/2469-6714.106
Copyright: © 2015 Muiño-Mosquera L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access