Abstract
A growing body of scientific literature has been showing the importance of measuring the serum levels of 25-OH Vitamin D (considered to be a reliable indicator of the overall Vitamin D status of a patient). Since the development of the first immuno-assays for 25-OH Vitamin D (over four decades ago), significant improvements have been made in the way 25-OH Vitamin D serum levels are measured. These improvements are, however, still lagging when compared to the exponential demand for Vitamin D testing in clinical settings. Indeed, current 25-OH Vitamin D assays are still slow, have low throughput and are still performed by a limited number of clinical laboratories. In this article, we report on a rapid technique to measure serum 25-OH Vitamin D that uses only two reagents and that can be adapted to virtually any automated spectrophotometric chemistry analyzer. The new technique uses polystyrene (latex) nanoparticles that have been sensitized with Vitamin D-specific antibodies. Extensive performance testing of this new 25-OH Vitamin D assay (namely the Diazyme EZ Vitamin D Assay) has been performed on one of the most common clinical chemistry analyzer (the Beckman AU680). Results show that the assay is sensitive, precise, linear, accurate and fast (throughput over 600 tests per hour). The assay has received approval for clinical use by the US Food and Drug Administration (FDA) and is certified (for accuracy and precision) by the Center for Disease Control and Prevention (CDC).
Highlights
- Two-reagent nanoparticle-based 25-OH Vitamin D assay for general chemistry.
- Assay has been validated for use on virtually all ubiquitous chemistry analyzers.
- With a throughout of at least 600 tests/hour, the assay aims at making Vitamin D testing routine and affordable.
Keywords
Vitamin D; Immuno-turbidimetry; Nanoparticles; EZ Vitamin D Assay
Abbreviations
25-OH Vitamin D: 25-hydroxy Vitamin D; VDBP: Vitamin D Binding Protein; 1, 25-(OH) 2 Vitamin D: 1, 25-dihydroxy Vitamin D; HPLC: High-Performance Liquid Chromatography; RIA: Radio Immuno Assay; LC-MS/MS: Liquid Chromatography Tandem Mass Spectrometry; CLIA: Chemiluminescent Immuno Assay; EIA: Enzyme Immuno Assay; DEQAS: Vitamin D External Quality Assessment Scheme
Introduction
Vitamin D is a steroid-derivative that exists in several forms that include Vitamin D2 (Ergocalciferol) and Vitamin D3 (Cholecalciferol) [1]. Humans cannot synthesize Vitamin D2 and rely on intakes from certain foods and Vitamin D supplements. Vitamin D3 is synthesized by the human skin [1-4]. The active form of Vitamin D (1, 25-(OH) 2 Vitamin D) is produced through two successive, enzyme-catalyzed, hydroxylation reactions: one in the liver (producing 25-OH Vitamin D) and one in the kidneys (producing 1, 25-(OH) 2 Vitamin D) [2].
There is a general consensus among scientists that levels of 25- OH Vitamin D (the total amount of 25-OH Vitamin D2 and 25-OH Vitamin D3, also known as total 25-OH Vitamin D) is the most accurate clinical marker for the overall Vitamin D status of a patient [5- 7]. Furthermore, a large and exponentially growing body of scientific research has been linking Vitamin D deficiency to various diseases and physiological disorders that include bone disorders, autoimmune diseases, diabetes, infectious diseases, cardiovascular diseases and cancer [5-12]. In addition, because experimental evidence appears to show a link between polymorphism in Vitamin D receptor genes and instances of osteoarthritis and disc degeneration in certain patients, Vitamin D appears to also play a role in these medical conditions [13]. As a result, regular Vitamin D screening has been recommended since the early 2000s by several institutions such as the American Endocrine Society, which explains that patients suffering from obesity or osteoporosis as well as those receiving long-term corticosteroids treatments or anti-seizure medications are at high risk for Vitamin D deficiency and should therefore be tested for Vitamin D regularly [7-10].
Current 25-OH Vitamin D testing methodologies are limited to LC-MS/MS, CLIA, HPLC and EIA [14-23]. Although these methodologies have improved significantly and can now be automated, their throughput still remains low. In addition, these methodologies often require dedicated equipment and highly trained personnel (i.e. LC-MS/MS). As a result, the number of clinical laboratories that has the ability to perform in-house Vitamin D testing is still lagging. This is particularly due to the fact that Vitamin D testing has not been possible on general clinical chemistry analyzers (used to run routine chemistry tests such as glucose, cholesterol and the like). Because of these limitation, the Vitamin D test is still a specialty test (only be performed by a limited number of laboratories) and is still costly for patients, laboratories and insurance companies.
In this article, we report on the development of a simple-to-use, two-reagent, 25-OH Vitamin D assay that has been specifically designed to run on virtually any general chemistry analyzer. Because of its high throughput and instrument-independent performance, this nanoparticle-base assay is poised to make Vitamin D testing more common among clinical chemistry laboratories and more affordable to all stakeholders.
Materials and Methods
Raw materials
Chemicals for nanoparticle conjugation and reagent formulation were obtained from Sigma-Aldrich. Vitamin D derivatives and crossreactants were obtained from Cerilliant. Biological specimens were obtained from ProMedDx.
Equipment
Performance of the assay was extensively tested on the Beckman AU680 analyzer (master analyzer). Performance was further validated on series general chemistry analyzers that include Roche Modular P, Roche Cobas c501, Roche Cobas c701 and Abbott Architect c8000.
Reagents, calibrators and controls
The assay kit (namely EZ Vitamin D Assay) consists of two reagents (namely R1 and R2), a set of five calibrators and a set of two controls. All reagents, calibrators and controls are ready to use and liquid-stable at 2-8°C until the expiration date shown on the bottle/ vial. Reagent R1 contains crowding agents, proprietary stabilizers and preservatives. Reagent R2 contains nanoparticles conjugated to anti-25-OH Vitamin D antibodies and anti-25-OH immunecomplex antibodies, proprietary stabilizers and preservatives. Calibrators and controls are manufactured from normal pooled human serum and contain stabilizers and preservatives to meet shelflife requirements.
Assay sequence on the beckman AU680 analyzer
The fully automated assay sequence performed by the Beckman AU680 is described as follows. The assay starts with the sequential addition of 3 µL of serum sample and 160 µL of reagent R1 followed by mixing and incubation at 37°C for 4 min. Reagent R2 (40 µL) is then added and mixed, triggering an agglutination signal that is measured at 700 nm.
Assay performance and validation
The assay was evaluated according to the Clinical and Laboratory Standards Institute (CLSI) approved standards and guidelines: CLSI EP5-A2, CLSI EP6-A, CLSI EP7-A2, CLSI EP9-A3 and CLSI C28-A3 for, respectively, precision, linearity, interference, method comparison and reference range. Data was processed with the EP Evaluator software (version 11.0) and Excel (Microsoft).
Results
Assay design and dose-response
For an assay to run on a general chemistry analyzer, it has to have very few reagents (preferably two) and should generate a reliable 25- OH Vitamin D dose-response within 10 min. This was achieved by designing two reagents, namely R1 and R2. A given patient serum sample is first mixed with Reagent R1, which contains releasing agents that dissociate the 25-OH Vitamin D moiety from its serum transporter (Vitamin D Binding Protein). Upon the addition of Reagent R2, the crowding agents present in Reagent R1 promote the 25-OH Vitamin D-specific agglutination of nanoparticles. Specific and dosedependent agglutination is mediated by two types of nanoparticles present in Reagent R2. The first type of nanoparticles is coated with an anti-25-OH Vitamin D antibody. The second type of nanoparticles is coated with a proprietary antibody that binds to the immunecomplex thus formed. As such, the presence of 25-OH Vitamin D in the reaction cuvette triggers a cascade of sequential antibody bindings that lead to nanoparticle agglutination. The extent of this agglutination is proportional to the amount of 25-OH Vitamin D in a patient’s serum sample. A typical dose-response curve (monitored by light scattering at 700 nm) is shown in figure 1.
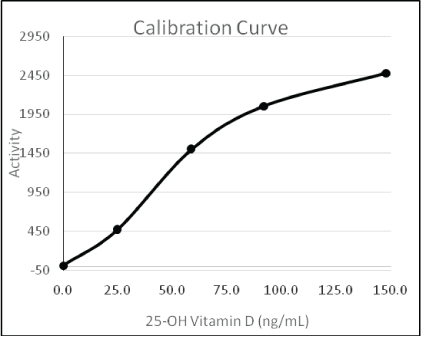
Figure 1: Typical 25-OH Vitamin D calibration curve on the Beckman AU680.
The y-axis labelled activity corresponds to the optical density measured at 700 nm multiplied by a factor of 104. The x-axis denotes the concentration of 25-OH Vitamin D in ng/mL.
Assay Performance on the Beckman AU680 Analyzer
Precision: A complex precision study was performed on two serum controls and nine serum samples (spanning the dynamic range of the assay), using three independent reagent lots. Measurements were done in duplicates, twice a day, over the course of 20 days. A total of 240 data points were obtained for each serum sample. Statistical analysis of the precision data is shown in table 1. Results show that the obtained within-run, between-run, between-day, between-lot and total percentage CV (for the three lots of reagents tested) were less than 10% for samples ranging between 15 and 147.8 ng/mL and less than 20% for samples ranging between 7.6 and 15 ng/mL. These results demonstrate a very precise assay over a wide dynamic range.
|
Sample |
Mean |
Within-Run |
Between-Run |
Between-Day |
Between-Lot |
Total |
| (N=240) |
SD |
%CV |
SD |
%CV |
SD |
%CV |
SD |
%CV |
SD |
%CV |
| Control 1 |
21.7 |
0.9 |
3.90% |
0.6 |
2.80% |
0.8 |
3.80% |
1.3 |
5.90% |
1.3 |
6.20% |
| Control 2 |
42.5 |
1 |
2.40% |
0.8 |
2.00% |
1.1 |
2.50% |
1.4 |
3.30% |
1.7 |
3.90% |
| Sample 1 |
11.1 |
0.9 |
8.30% |
0.5 |
4.40% |
1.5 |
13.70% |
1.9 |
16.80% |
1.8 |
16.60% |
| Sample 2 |
18.2 |
0.9 |
4.90% |
0.7 |
3.90% |
1.1 |
6.00% |
1.6 |
8.60% |
1.6 |
8.70% |
| Sample 3 |
22.1 |
0.8 |
3.80% |
0.8 |
3.80% |
0.4 |
1.80% |
1.2 |
5.60% |
1.2 |
5.60% |
| Sample 4 |
42.8 |
0.9 |
2.00% |
1 |
2.40% |
0 |
0.00% |
1.4 |
3.20% |
1.3 |
3.10% |
| Sample 5 |
59.5 |
1 |
1.70% |
0.7 |
1.20% |
1 |
1.70% |
1.6 |
2.70% |
1.6 |
2.70% |
| Sample 6 |
80.2 |
1.3 |
1.60% |
1.1 |
1.40% |
1.1 |
1.40% |
2 |
2.50% |
2 |
2.50% |
| Sample 7 |
99.5 |
1.8 |
1.80% |
1.5 |
1.60% |
1.3 |
1.30% |
2.7 |
2.70% |
2.7 |
2.80% |
| Sample 8 |
117.6 |
2.2 |
1.90% |
2 |
1.70% |
2.2 |
1.90% |
3.7 |
3.10% |
3.7 |
3.20% |
| Sample 9 |
139.2 |
2.7 |
1.90% |
2.6 |
1.80% |
1.7 |
1.20% |
4 |
2.90% |
4.1 |
2.90% |
Table 1: Statistical Analysis of the Complex Precision Study.
Linearity: To assess linearity, a human serum sample was spiked with a 25-OH Vitamin D stock solution to an average concentration of 153.0 ng/mL (as measured in triplicates using the Diazyme EZ Vitamin D assay). This high sample was then diluted with a low 25-OH Vitamin D serum sample (averaging 4.7 ng/mL) to create 11 equally distributed linearity levels. These 11 levels were measured in triplicates. Linear regression of the obtained results is plotted in figure 2 and shows that the assay is linear up to 153.0 ng/mL.
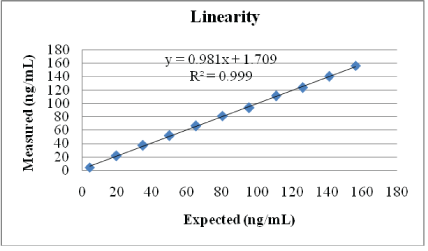
Figure 2: Linearity test.
A high 25-OH Vitamin D was blended with a low 25-OH Vitamin D sample to create 11 linearity levels. Measurements were done in triplicates.
Sensitivity: To determine the Limit of Quantification (LOQ) of the assay, five 25-OH Vitamin D serum samples were diluted with 25-OH Vitamin D depleted serum and measured in 40 replicates over five days. The LOQ was determined by plotting the percentage CV for the five samples versus mean concentrations and fitting the curve to the 95% confidence interval at a CV of 20% (figure 3). Results show that the LOQ of assay is as low as 4.3 ng/mL.
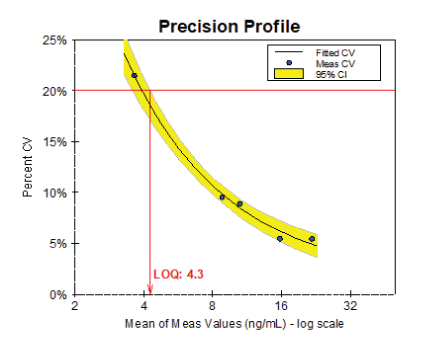
Figure 3: Determination of the Limit of Quantitation.
Method comparison: For method comparison, individual serum samples were tested with the Diazyme EZ Vitamin D assay on Beckman AU680 analyzer and compared to a previously approved predicate device (k133410). A total of 171 samples (spanning the assay’s AMR) were used. These samples included 5 very low samples (below 15 ng/ mL) and 5 very high samples (above 135 ng/mL, obtained by spiking 25-OH Vitamin D into individual serum samples). Total percent of altered samples in this method comparison study was 9.4%. All measurements were done in singlet’s. Linear regression of the results is plotted in figure 4 and shows that the newly developed assay is accurate and substantially equivalent to a commercially available assay.
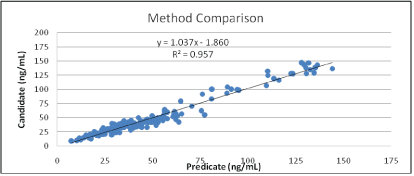
Figure 4: Method comparison.
171 serum samples were measured with an enzymatic assay for 25- OH Vitamin D (predicate, k133410) and with the newly developed assay (candidate).
Matrix comparison: To evaluate the effect of anticoagulants, the Diazyme EZ Vitamin D assay was used to measure the 25-OH Vitamin D concentrations of matched sets of serum, K2-EDTA plasma, K3- EDTA plasma and Li-Heparin plasma. The reported values for each sample and for each matrix were obtained from single measurements on Beckman AU680 analyzer. The total number of matched sets tested was 54. Results are presented in figure 5A-5C. Results show that the newly developed assay is suitable for use with human specimen consisting of serum, K2-EDTA plasma, K3-EDTA plasma or LiHeparin plasma.
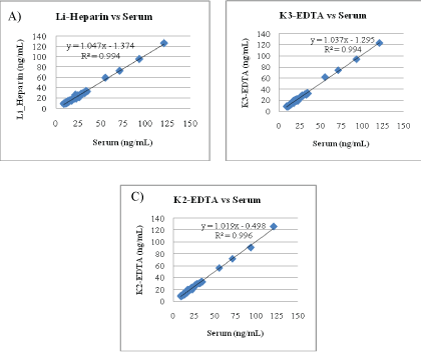
Figure 5: Matrix comparison.
(A) Li-Heparin plasma versus serum.
(B) K3 -EDTA-Plasma versus Serum.
(C) K2 -EDTA-Plasma versus Serum.
Reference interval: To determine a reference interval of the assay, the 25-OH Vitamin D serum concentrations of a US population of 145 apparently healthy individuals were measured. 47 samples were collected from Pennsylvania (Northern U.S.), 49 samples were collected from Tennessee (Central U.S.) and 49 samples were collected from Texas (Southern U.S.). Participants were selected based on age (21-67 years old), not taking any Vitamin D supplements, no history of parathyroid or calcium regulatory diseases and no intake of any medications that are known to affect absorption or catabolism of Vitamin D, including cholesterol absorption inhibitors such as Vytorin®, Inegy™ or Zetia); anticonvulsants such as Neurontin, Depakine® and Trileptal; glucocorticoids such as Cortisol, Prednisone and Dexamethasone; HAART (AIDS treatment) or anti-rejection medications.
Non-parametric statistics representing the central 95% of the population are shown in figure 6. The reference interval of the assay is established between 7.2 ng/mL and 41.6 ng/mL. This interval is consistent with the published scientific literature [24].
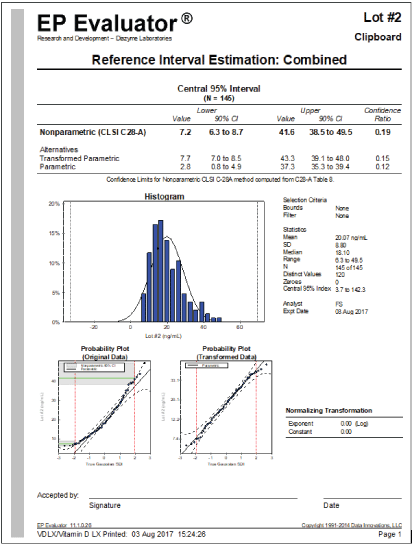
Figure 6: Reference Interval Estimation.
Stability: Accelerated stability of reagents, calibrators and controls was assessed at 37°C for 22 days and showed that the assay still performed as expected in terms of precision, sensitivity, linearity and sample recovery (see figure 7). Based on these results the shelf life of the assay kit can be up to 24 months at 2-8°C. Figure 8 shows that sample recovery of a 13-month old assay kit is substantially equivalent to that of the freshly manufactured kit.
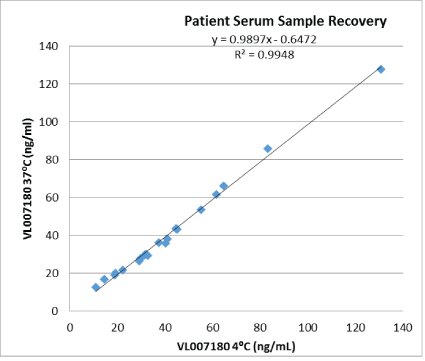
Figure 7: Accelerated stability of reagents.
The (y) axis denotes measurement done with a heat-stressed reagent (22 days at 37°C). The (x) axis denotes measurements done with reagents kept at 2-8°C. Real-time stability of the assay kit (at 2-8°C) is on-going.
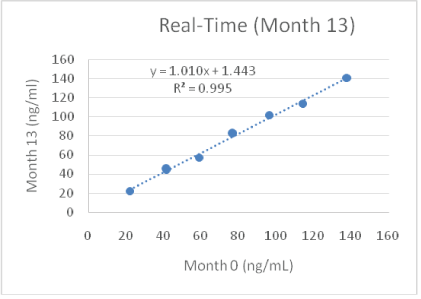
Figure 8: Real-time stability of reagents.
8 serum samples were measured with freshly manufactured reagents (x-axis, month 0). The same serum samples (kept frozen) were measured 13 months later with the same assay kit (y-axis).
Throughput: To estimate the throughput of the assay, serum samples were loaded in batch mode on the AU680 analyzer and the number of serum sample results produced measured at 30 min and 45 min. Throughput was extrapolated to one hour by averaging the results at 30 min and 45 min. The assay was found to be the fastest 25- OH Vitamin D, producing 625 test results per hour on the Beckman AU680 analyzer.
Validated analyzers: In order to assess the versatility of the newly developed 25-OH Vitamin D kit, its performance was evaluated and validated on different chemistry analyzers. Table 2 lists the analyzers for which the assay was fully validated (data not shown) and shows that the assay can be run on at least 13 different general chemistry analyzers made by 6 different manufacturers. This versatility is important as it brings 25-OH Vitamin D testing capabilities to virtually any clinical chemistry laboratory.
| Analyzer Name |
Manufacturer |
| AU680 |
Beckman |
| AU400 |
Beckman |
| AU5800 |
Beckman |
| Architect c4000 |
Abbott |
| Architect c8000 |
Abbott |
| DZ-Lite c270 |
Diazyme |
| Modular P |
Roche |
| Cobas c501 |
Roche |
| Cobas c701 |
Roche |
| Cobas c702 |
Roche |
| Hitachi 917 |
Roche |
| Pentra 400 |
Horiba |
| Biolis 30i |
Tokyo Boeki |
Table 2: List of Analyzers Validated for the EZ Vitamin D Assay.
Discussion
25-OH Vitamin D testing has been evolving over the past several decades. From the earliest methods developed in the 1970s (ELISA and RIA) to the development of liquid chromatography, mass spectrometry and chemiluminescence methods, Vitamin D testing has gradually improved in accuracy, precision, automation and throughput [25-26]. At the same time, clinical evidence for the importance of monitoring 25-OH Vitamin D levels has risen exponentially, creating more need for an easy, fast, accurate and costeffective method for 25-OH Vitamin D [25].
The nanoparticle-enhanced assay described in this article presents the latest development in the detection of 25-OH Vitamin D. Using simple photometric detection (as opposed to Chemiluminescence and other complicated technology such as RIA, HPLC and LC-MS) and consisting of only two reagents, the newly developed assay represents a significant leap towards providing Vitamin D testing capabilities to virtually all clinical chemistry laboratories. Traditional Vitamin D methodologies require either highly trained personnel (HPLC, LC-MS and RIA) or special analyzers for chemiluminescence detection. These requirements are often absent in small or medium clinical chemistry labs who end up contracting their Vitamin D testing to major lab testing corporations. This delays patients’ results and increases costs. With the new Vitamin D assay described in this article, any clinical chemistry lab can perform in-house Vitamin D testing on any ubiquitous chemistry analyzer.
Furthermore, because of traceability and harmonization concerns with other 25-OH Vitamin D methodologies, the current 25-OH Vitamin D assay is traceable to the National Institute of Standards and Technology (NIST) standard SRM972a and is currently certified by the Centers for Disease Control and Prevention (CDC) Vitamin D Standardization-Certification Program (VDSCP). The assay is also enrolled to two third-party Proficiency Testing (PT) programs: the UK-based Vitamin D External Quality Assessment Scheme (DEQAS) and the US-based College of American Pathologists Accuracy Based Vitamin D (CAP ABVD).
Considering the importance of 25-OH Vitamin D testing in preventive medicine, we believe that the newly developed Vitamin D assay will greatly contribute to the “democratization” of Vitamin D testing. This will ultimately contribute to improving patients’ health outcomes and decreasing healthcare costs in the US and abroad.
Conclusion
This article reports on a new method for the detection of 25-OH Vitamin D in human serum and plasma. The method, named EZ Vitamin D, is an immune turbidimetric assay whereby the patient’s 25-OH Vitamin D levels are assessed by measuring the extent of agglutination of nanoparticles coated with specific Vitamin D antibodies. The performance of these simple two-reagent, spectrophotometric assay for Vitamin D has been successfully and extensively tested on several automated chemistry analyzers. The assay has been cleared for clinical use by the US FDA and certified by the CDC.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.