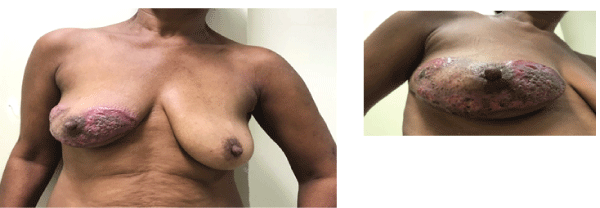
Figure 1: Images (A and B) of the right breast at first consultation. Extensive and irregular ulceration area with raised borders predominantly in purple color sparing areola and nipple.


Andressa Gonçalves Amorim* Marcellus Nascimento Moreira Ramos Felipe Andreotta Cavagna Maria Isabela Bloise Alves Caldas Sawada Alexandre Santos Melitto Gabriella Mazzarone Andre Mattar Jorge Yoshinori Shida Luiz Henrique Gebrim
Women’s Health Center, Pérola Byington Hospital, São Paulo, Brazil*Corresponding author: Andressa Gonçalves Amorim, Women’s Health Center, Pérola Byington Hospital, São Paulo, Brazil, Tel: +55 11 998573787; E-mail: andressaamorim88@hotmail.com
Pyoderma gangrenosum is an inflammatory, ulcerative skin disorder characterized at histopathology by the presence of neutrophils in the skin. The condition may either develop spontaneously or in association with surgical trauma or systemic or neoplastic diseases. Clinical presentation varies, but is basically characterized by ulcerated, painful, dark red or purple skin lesions with irregular, inflamed and raised borders and a necrotic base, with single or multiple small abscesses. This report refers to a patient with spontaneous pyoderma gangrenosum, with lesions on her right breast, left axilla, left eyelid and chin. The patient responded well to systemic corticosteroids.
Breast; Pyoderma gangrenosum; Neutrophilic infiltrate; Ulcer
Pyoderma gangrenosum is a rare disease, with an annual incidence estimated at 3-10 cases per million inhabitants [1]. Although individuals of any age can be affected, including children [2] the disease is more common in adults, with the mean age of patients being between 40 and 60 years [3]. Women are more likely to be affected than men [4]. Louis Brocq first described the condition in patients who developed ulcerated lesions, while Bursting et al. reported a series of cases of inflammatory ulcers in patients with pathergy [5].
This inflammatory and ulcerative skin disorder is characterized at histopathology by the presence of neutrophils in the skin. Onset generally involves the rapid development of one or more painful, purulent ulcers with irregular borders on normal or injured areas of the skin [6]. Pyoderma gangrenosum of the breast presents with atypical clinical signs and is characterized by an exclusion-based diagnosis.
The institute’s internal review board approved the study protocol under reference number 2.619.721. The patient signed an informed consent form.
A 51-year old, hypertensive black woman, who reported being a current smoker, drinker and user of illicit drugs (crack addict), presented with a complaint of pruritus on her right breast that had been present for the preceding six months. The patient went on to develop a very painful, ulcerated lesion with irregular, undermined, violet borders and a necrotic and purulent base. The nipple areola complex remained unaffected (Figures 1A-1B). Around two months later, painful, ulcerated lesions appeared on her left axilla, left eyelid and chin (Figures 2A-2C).

Figure 1: Images (A and B) of the right breast at first consultation. Extensive and irregular ulceration area with raised borders predominantly in purple color sparing areola and nipple.
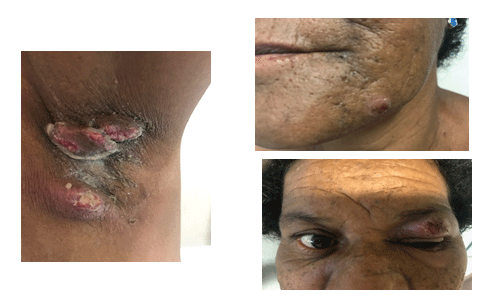
Figure 2: Lesions on the left axilla (A), chin (B) and left eye(C). Pustule lesion containing small abscesses.
The lesions were initially interpreted as an infection; hence, systemic antibiotic therapy was started. Culture of the material was performed and the lesion on the right breast was biopsied. The condition deteriorated noticeably, with an increase in the size of the areas affected. Histopathology of fragments from the patient’s right breast revealed an intense, diffuse, perivascular, neutrophilic and lymphocytic inflammatory infiltrate in the skin.
The patient complained of moderate tiredness, although there were no other signs or symptoms of systemic disease. No abnormalities were found in full blood count, erythrocyte sedimentation rate, Thyroid-Stimulating Hormone (TSH), Free Thyroxine (free T4), or fasting glucose levels. Chest, abdominal and pelvic tomographies were normal. Rheumatologic tests were normal except for rheumatoid factor>120 (normal value <40) and Antinuclear Factor (ANF) reactive/dense fine speckled nuclear pattern 1/640 (normal value: <1/80).
The patient initiated treatment with oral prednisone 40 mg/day. Aqueous antiseptic soaps were used daily to clean the lesions and an aqueous gel of sodium alginate was applied twice daily, which relieved the pain. The ulcers improved substantially after 90 days of corticosteroid therapy, with hyperchromic atrophic and cribiform scarring (Figures 3A-3D).
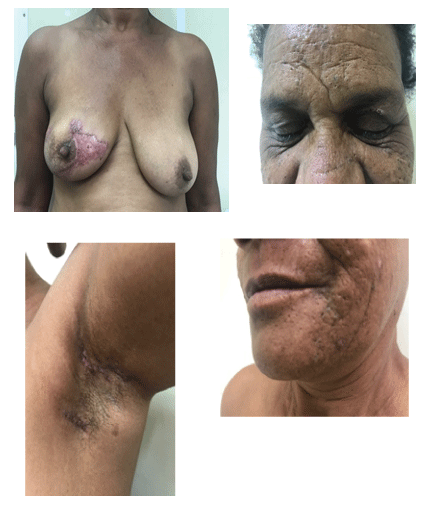
Figure 3: 90 days of glucocorticoid showing good response to systemic corticosteroid therapy with regression of the inflammatory signs. The skin is almost normal (A,B,C and D).
Pyoderma gangrenosum is characterized by a predominantly neutrophilic infiltrate in the skin and is classified as a neutrophilic dermatosis. Nevertheless, the etiology of pyoderma gangrenosum has yet to be clarified.
Hypotheses regarding the pathogenesis of this disease included occult bacterial infection, circulating auto antibodies and the Shwartzman reaction [7]. However, subsequent studies found little support for these theories. Therefore, abnormalities in neutrophil function, genetic variations and dysregulation of the innate immune system are now considered the principal factors involved in the pathogenesis of pyoderma gangrenosum [8,9].
Neutrophils are the principal cells in pyoderma gangrenosum, and good patient response to drugs that suppress integrin-mediated neutrophil adherence function, such as dapsone, suggests an important role for these cells. Furthermore, abnormalities in the behavior of neutrophils, which could have been related to aberrant intracellular metabolic oscillations, were detected in a patient with pyoderma gangrenosum [9].
Genetic susceptibility may contribute to the development of pyoderma gangrenosum. Familial cases of the disease have been reported [10]. In addition, the PAPA syndrome (OMIM # 604416), an autosomal dominant disorder characterized by pyogenic sterile arthritis, pyoderma gangrenosum and acne, has been associated with mutations in the PSTPIP1/CD2BP1 gene at chromosome 15q. This gene codifies proline-serine-threonine phosphatase-interacting protein 1 (also known as CD2 antigen-binding protein 1). Mutations in this gene may result in inflammation [11,12].
Pyoderma gangrenosum generally occurs in association with other disorders such as inflammatory bowel disease and arthritis, suggesting that immune system dysregulation may contribute towards development of the disease [9]. The increased production of cytokines such as Interleukin (IL)-8 and IL-23, which are involved in leukocyte function, may constitute a relevant factor [13,14].
The clinical presentation of pyoderma gangrenosum may differ from case to case; however, it is generally characterized by painful, ulcerated, single or multiple skin lesions [15]. The lesion has red or purple, irregular, inflammatory, raised borders and a necrotic base containing small abscesses. The ulcerated lesions may produce a purulent, hemorrhagic exudate. The peripheral growth of the lesions is serpiginous and results from excavation of the borders or the appearance of new hemorrhagic pustules. The superficial ulcers may be confined to the dermis alone; however, in general they extend to the panniculus adiposus and as far as the underlying fascia. The lesions are generally single; however, coalescence may be seen [16].
The site of the lesions varies, most commonly including the lower limbs, abdomen, upper limbs, breasts, buttocks, genitalia and face [17]. The most common extracutaneous clinical manifestations occur in the lungs, joints and digestive tract, and may be related to the generalized inflammatory process. Pulmonary involvement may lead to the appearance of pleural effusion, nodules, cavitations, bronchial pneumonia and abscesses. Other abnormalities may be found in the eyes (necrotizing sclerokeratitis), liver (hepatitis, granulomas), spleen, bones and bone marrow (redblood cell, leukocyte and platelet disorders) [18-20].
Two different clinical patterns of progression are associated with the initial course of pyoderma gangrenosum:
There are no specific laboratory tests or pathognomonic histologic characteristics with which to confirm diagnosis. Therefore, the diagnosis of pyoderma gangrenosum depends exclusively on observation of its clinical characteristics and course [16].
The differential diagnosis for pyoderma gangrenosum includes important diseases, most of which progress to skin ulcers: bacterial skin infections (folliculitis, furunculosis, carbuncles, ecthyma, gangrenous cellulitis, necrotizing fasciitis), sexually transmitted diseases (syphilis, donovanosis, cancroid), mycobacteriosis, accidents involving animals and insect bites, leishmaniasis, deep mycoses (sporotrichosis, cryptococcosis, paracoccidioidomycosis, chromomycosis, mucormycosis, histoplasmosis, Rhizopus and Aspergillus infections), amebiasis, herpes simplex, small-vessel vasculitis, Wegener’s granulomatosis, polyarteritis nodosa, erythema multiforme, pustular psoriasis, pustular drug eruption, autoimmune bullous diseases, venous ulcers, arterial ulcers, antiphospholipid syndrome, specific thrombopathies, Sweet’s syndrome, Behçet’s disease, calciphylaxis, psychodermatoses, and primary and metastatic skin neoplasms [22,23].
Primary infectious ulcers may be the result of bacterial infections, fungal infections, spirochete infections or protozoan infections, either resulting from direct inoculation or systemic dissemination:
Vascular disorders may result in ulcers on the lower limbs through inflammatory processes that cause vasculitis or occlusion of the vessel, leading to ischemia:
Systemic corticosteroid therapy is the most effective treatment for pyoderma gangrenosum [4]. Corticosteroid therapy can halt progression of the ulcerations and prevent the development of new lesions. High initial doses are required in most cases, around 100-200 mg/day of prednisolone or 60-80 mg/day of prednisone [26]. Prognosis is generally good, particularly for those patients who respond rapidly to the initial treatment regimens [27].
The clinical course of pyoderma gangrenosum can be unpredictable and highly variable from onset and throughout progression of the condition; hence diagnosis of this pathology may constitute a challenge. Some patients develop painful lesions that grow progressively, accompanied by fever. In others, lesions are chronic, with ulcerations that progress slowly. Pathogenesis remains to be fully clarified. Recent progress in the field of immunogenetics suggests the availability of more accurate predictive tests in the future, since there is currently no specific test on which diagnosis can be based. Since diagnosis is reached exclusively on the basis of clinical characteristics, it is very important to take this disease into consideration when evaluating differential diagnoses.
None
None declared.
Download Provisional PDF Here
Aritcle Type: CASE REPORT
Citation: Amorim AG, Ramos MNM, Cavagna FA, Sawada MIBAC, Melitto AS, et al. (2018) Pyoderma Gangrenosum: Report of a Case Involving the Breast. J Clin Cosmet Dermatol 2(3): dx.doi.org/10.16966/2576-2826.133
Copyright: © 2018 Amorim AG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access