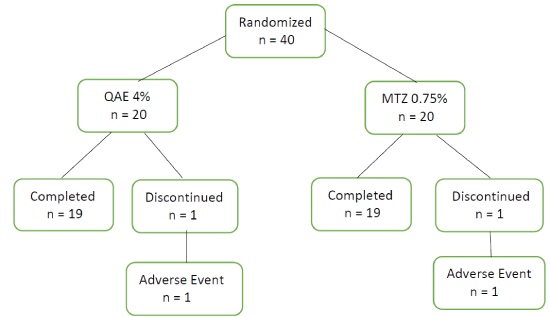
Figure 1: Study population and causes for withdrawal


Christian Diehl1* Alicia Ferrari2
1Universita Degli Studi Guglielmo Marconi, Rome, Italy*Corresponding author: Christian Diehl, Universita Degli Studi Guglielmo Marconi, Rome, Italy, Tel: +54 0351 480 3111; E-mail: chdiehl@hotmail.com
Background: Rosacea is a chronic inflammatory skin disease with few therapeutic options. The efficacy of topical Quassia amara 4% was assessed on rosacea in a previous open trial but not compared to any other currently accepted therapy.
Objective: This trial aimed to compare the efficacy and tolerance of a Quassia amara 4% cream with a Metronidazole 0.75% cream in patients with various grades of rosacea.
Methods: In this randomized, double-blinded, parallel-group study, patients with various grades of rosacea were assigned to Quassia amara 4% (QA 4%) cream or to metronidazole 0.75% (MTZ 0.75%) cream twice daily over 6 weeks. Efficacy assessments were a biweekly evaluation of flushing, erythema, telangiectasiae, papules and pustules counts and global assessment taking into account these five parameters. Safety assessment included incidence of adverse events (AEs) and local tolerance.
Results: A total of 40 patients were equally randomized in two groups to receive QA 4% or MTZ 0.75%. At week 6, QA 4% was significantly superior to MTZ 0.75% in terms of reduction from baseline in flushing and telangiectasiae, and similar in terms of improvement of erythema and reduction of papules and pustules. The global assessment showed improvement as soon as the second week of treatment with QA 4% whilst only from the 4th week onward with MTZ 0.75%. As regards tolerance, one patient in QA 4% group suffered AE under the form of allergic conjunctivitis not related to the use of the product and one patient in MTZ 0.75% group experienced a severe contact allergy to the product.
Conclusion: Our data implicated that QA 4% cream is superior to MTZ 0.75% cream in the management of flushing and telangiectasiae and the activity of both products is similar as regards improvement of erythema and reduction of the number of papules and pustules. Both products were well-tolerated by our patients in the course of this study.
Rosacea; Quassia amara; Double-Blinded Trial; Erythema; Telangiectasiae; Papules; Pustules
Rosacea is a chronic inflammatory skin condition affecting mostly women aged 30 to 50, and usually worsens along the years. Epidemiologic studies in rosacea provide widely varying prevalence estimates. Beyond actual differences in disease prevalence, this variation may be a result of differences in case ascertainment, study design, environmental factors, and population phototype [1]. In Europe and the USA, prevalence ranges from less than 1% to more than 22% [2]. Rosacea has long been named “Curse of the Celts” as it is thought to affect more likely individuals with phototypes I or II. However, we personally think that we should review this sentence, as rosacea is also very frequent in patients with phototype III and can be observed, although more rarely, in darker ones. The hallmark of rosacea is central facial persistent erythema, typically affecting the cheeks, chin, forehead, and nose while sparing the perioral and periocular regions [3]. This feature has been considered to be the sole requisite criterion for the diagnosis of rosacea [3]. In 2002, the National Rosacea Society assembled a committee to develop a standard classification system that can serve as a diagnostic instrument to investigate the manifestations and relationships of the several subtypes and potential variants of rosacea [4]. Subtype 1: Erythematotelangiectatic rosacea (ETR) is mainly characterized by flushing and persistent central facial erythema. Subtype 2: Papulopustular rosacea (PPR) is characterized by persistent central facial erythema with transient papules or pustules or both in a central facial distribution. Subtype 3: Phymatous rosacea includes thickening skin, irregular surface nodularities, and enlargement; rhinophyma is the most common presentation. Subtype 4 represents ocular rosacea. The pathogenesis of rosacea is not yet completely understood. Its aetiology is multifactorial including exogenous factors such as UV light and it maybe secondary to parasitic involvement, particularly Demodex folliculorum mites. Such factors activate neurovascular and/or immune responses and consequently inflammatory cascades. A recent Cochrane review noted that it is unclear which treatment is most effective, but some evidence supports the efficacy of topical metronidazole, azelaic acid and subantimicrobialdose doxycycline in the treatment of moderate to severe rosacea [5].
Metronidazole (MTZ) is an antibiotic and antiprotozoal nitroimidazole component. It also appears having anti-inflammatory and antioxidant activity.
Quassia amara features a shrub or small member of the family Simaroubaceae originating from South America. In this region (namely Amazon rainforest, Suriname, French Guyana or Peru, Quassia is widely used in much in the same manner as quinine bark, for malaria and fevers. Another wide indication for Quassia in folk medicine is anorexia: it is used as a bitter stomachic to stimulate appetite and digestion, by increasing the secretion of digestive juices. It is also widely used as an antiparasitic, especially in diarrheal and dysentery caused by amoeba in Mexico, Brazil or Guyana. The insecticide properties of Quassia have also to be highlighted, as boiling pieces of wood of Quassia in water; one obtains a spray effective against many insects. Chemically, it contains high levels of active phytochemicals, including the triterpenoid quassinoids. Various biological activities are described in the literature, including anti parasitic activities against pediculosis [6,7] and anti-inflammatory properties [8]. We had previously demonstrated the efficacy of topical Quassia amara extract (QAE) in the treatment of rosacea [9].
As there was no comparative study of the activity of QAE on rosacea vs. a widely accepted treatment such as MTZ, we have designed and performed this study.
This was a randomized, double-blinded, parallel-group study, comparing the efficacy and safety of QAE 4% cream vs. MTZ 0.75% cream over a period of 6 weeks. The study took place in Santa Catarina Dermatological Clinic, Cordoba, Argentina, from July 2015 to November 2015. Study visits were as follows: a screening visit at D0, and at D14, D28 and D42.
The QAE 4% cream used in this study was prepared from an extract issued from the maceration in a water/propylene glycol solvent of Quassia amara bark. The concentration of this extract in quassinoids is standardized at 1%, i.e. one gram of cream contains 0.4 mg of quassinoids. This product is protected by US Patent N° US 2014/0005259 A1.
Eligible subjects were 18 years or older, with Grade-I to IV rosacea with symptoms present for three months or more and not having received treatment for their rosacea during the month preceding their inclusion in the study in order to avoid any carryover effect of previous therapy. Exclusion criteria included known allergies to any component of the formula of both products under study, previous history of skin cancer (melanoma or non- melanoma) in affected areas, patient participation in other clinical studies within 3 months before this study, or patients whose mental condition did not permit good compliance.
40 patients were randomized (20 per group).
Prior to the start of the study, a randomization list was generated through a software using the method of randomly permuted blocks and was secured with restricted access. Treatment assignment was balanced in a 1:1 ratio and kit numbers were assigned sequentially in chronological order. The study design was double-blinded. The products were packaged in identical tubes, not allowing the investigator or the subject to know study treatments.
Subjects received according to randomization either QAE 4% cream or MTZ 0.75% cream, both twice daily at morning and bedtime as per labelling, during 6 weeks. Study drugs were to be applied in a thin layer on the lesions, avoiding the upper and lower eyelids, lips, eyes and mouth. The subjects were instructed to maintain a consistent lifestyle throughout the study, avoiding excessive sun exposure and known triggering factors. Interestingly, the study took place during the winter time, for this reason application of sun protector was not especially recommended to the patients, thus avoiding any bias in the results.
Treatment efficacy was assessed by counting the number of inflammatory papules and pustules rated as 0, no papule/pustule; 1, number of papules/ pustules <5; 2, number of papules/pustules >5 and <20; and 3, >20 and rating the flushing (0, no flush; 1, intermittent flushing; 2, permanent flushing; 3, intense flushing), erythema (0, no erythema; 1, mild erythema; 2, moderate erythema; 3, severe erythema), and telangiectasia (0, no; 1, mild; 2, moderate; 3, severe). Global assessment consisted in the sum of all five previous scores.
The model used was the generalized linear model log-linear Poisson, with logarithmic binding function, g (μ)=log (μ) and linear predictor η=μ0+αi, with α effect of view, i=1, 2.
From these models were obtained the estimations of odds ratios (OR) in order to interpret the correlation with time. When a parameter features a significant change from one visit to another, it is labelled as “significant marker” of efficacy in the treatment. These models were adjusted and estimated in Statistica 12 Software (Stat Soft Inc. 2015).
This study was conducted in accordance with the ethical principles derived from the Declaration of Helsinki and ICH (International Conference on Harmonization) Good Clinical Practices and in compliance with local regulatory requirements. All subjects provided a written informed consent before entering the study.
A total of 46 subjects were screened and 40 randomized to either QAE 4% (n=20) or MTZ 0.75% (n=20). 38 patients (95%) completed the study with one withdrawal in each group (Figure 1).

Figure 1: Study population and causes for withdrawal
Treatment groups were compared at baseline in terms of demographics and baseline disease characteristics (Table 1).
| QAE 4% (N=20) | MTZ 0.75% (N=20) | TOTAL (N=40) | |
| AGE (YEARS) | |||
| MEAN ± SD | 51.3 ± 14.1 | 54.1 ± 14.1 | 52.7 ± 14.2 |
| MIN-MAX | 18-77 | 36-74 | 18-77 |
| GENDER | |||
| FEMALE | 14 (70%) | 16 (80%) | 30 (75%) |
| MALE | 6 (30%) | 4 (20%) | 10 (25%) |
| SKIN PHOTOPYPE (FITZPATRICK) | |||
| I | 1 (5%) | 2 (10%) | 3 (7.5%) |
| II | 17 (85%) | 14 (70%) | 31 (77.5%) |
| III | 2 (10%) | 4 (20%) | 6 (15%) |
| GRADE OF ROSACEA | |||
| GRADE I | 1 (5%) | 0 | 1 (2.5%) |
| GRADE II | 11 (55%) | 15 (75%) | 26 (65%) |
| GRADE III | 7 (35%) | 5 (25%) | 12 (30%) |
| GRADE IV | 1 (5%) | 0 | 1 (2.5%) |
| GLOBAL ASSESSMENTAT BASELINE | 7.65 ± 3.26 | 7.2 ± 3.68 | 7.42 ± 3.48 |
| LOCALIZATION OF ROSACEA | |||
| FOREHEAD | 11 (55%) | 10 (50%) | 21 (52.5%) |
| MALAR | 19 (95%) | 20 (100%) | 39 (97.5%) |
| NOSE | 13 (65%) | 12 (60%) | 25 (62.5%) |
| CHIN | 9 (45%) | 11 (55%) | 20 (50%) |
Table 1: Demographic and baseline data
Regarding the first parameter under study, there was a significant decrease of flushing as soon as D14 with QAE 4% (mean score 1.55 vs. 1.8 at baseline [p=0.02]) and D42 (mean score 1.42, p>0.01) whilst there was no significant reduction with MTZ 0.75% (mean score at D42 1.53 vs. 1.75, p>0.05).
Erythema was significantly reduced with QAE 4% from D14 onward (mean score at baseline 2.3, at D14 2.1 [p<0.05) and at D42 1.47 [p<0.001). With MTZ 0.75% the reduction was significant from D28 onward (mean score at baseline 2.21, at D28 2.00 [p<0.05] and 1.84 at D42 [p<0.05]).
Telangiectasiae were reduced with QAE 4% as soon as D28 (mean score at baseline 1.85 vs. 1.32 at D28 [p<0.001]) whilst there were no significant results with MTZ 0.75% even at D42.
There was a significant reduction in the number of papules with both products from D28 onward (QAE mean score 0.89 at D28 vs. 1.25 at baseline [p<0.01)] and MTZ 1.00 at D28 vs. 1.15 at baseline [p<0.05]).
As regards to the number of pustules, similar results were observed. Mean score with QAE was 0.32 at D28 vs. 0.70 at D0 (p<0.01) and with MTZ 0.32 at D28 vs. 0.50 at baseline (p<0.05).
The global assessment was significantly improved with QAE as soon as D14 (mean score 6.85 vs. 7.65 at baseline [p<0.001]) on the contrary with MTZ this improvement was significant only at D28 (6.52 vs. 7.2 [p<0.01]) (Figure 2).
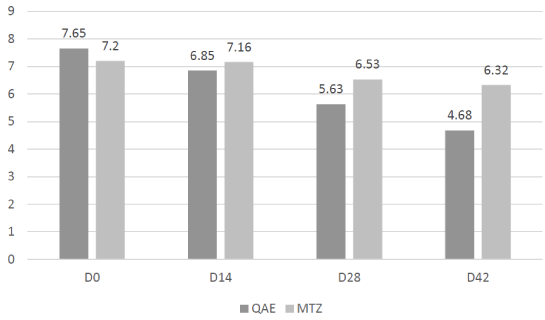
Figure 2: Evolution of Global Assessment score over the study
Detailed results of different scores are shown in table 2.
| QAE 4% | MTZ 0.75% | |||||||
| MEAN VALUES (SD) | D0 | D14 | D28 | D42 | D0 | D14 | D28 | D42 |
| FLUSHING | 1.80 ± 0.83 | 1.55 ± 0.69 | 1.42 ± 0.61 | 1.42 ± 0.61 | 1.75 ± 0.85 | 1.58 ± 0.69 | 1.53 ± 0.61 | 1.53 ± 0.61 |
| ERYTHEMA | 2.30 ± 0.47 | 2.10 ± 0.55 | 1.79 ± 0.63 | 1.47 ± 0.77 | 2.21 ± 0.63 | 2.10 ± 0.55 | 2.00 ± 0.58 | 1.84 ± 0.60 |
| TELANGIECTASIAE | 1.85 ± 0.81 | 1.70 ± 0.80 | 1.32 ± 0.88 | 1.00 ± 0.88 | 1.65 ± 0.88 | 1.63 ± 0.89 | 1.58 ± 0.84 | 1.53 ± 0.77 |
| PAPULES | 1.25 ± 1.02 | 1.15 ± 1.09 | 0.89 ± 0.87 | 0.68 ± 0.82 | 1.15 ± 1.09 | 1.21 ± 1.08 | 1.00 ± 0.94 | 0.95 ± 0.97 |
| PUSTULES | 0.70 ± 0.80 | 0.60 ± 0.82 | 0.32 ± 0.58 | 0.26 ± 0.56 | 0.50 ± 0.89 | 0.47 ± 0.90 | 0.32 ± 0.58 | 0.26 ± 0.56 |
| GLOBAL ASSESSMENT | 7.65 ± 3.34 | 6.85 ± 3.36 | 5.63 ± 2.67 | 4.68 ± 2.85 | 7.20 ± 3.78 | 7.16 ± 3.40 | 6.53 ± 2.87 | 6.32 ± 2.91 |
Table 2: Details of results (mean scores for each parameter at different endpoints of the study)
The incidence of adverse events was similar in both groups: one patient had to withdraw in each group. The patient from QAE group was suffering allergic conjunctivitis which did not appear to be related with the treatment. In MTZ group one patient withdrew because of a severe contact allergy directly related with the application of the product. In all other patients the tolerance was reported as excellent.
Topical MTZ at either 0.75% or 1% is probably the most commonly used therapy in the treatment of rosacea. The efficacy of MTZ on moderate-to severe rosacea has been assessed by various studies. The previously mentioned Cochrane review [5] states that in the fourteen trials included, topical MTZ was more effective than placebo and the results were both statistically significant and clinically important, disregarding the concentration used (0.75% or 1%). Upon separate analysis of these studies, the first finding is that the duration of the studies is usually longer than our study (two to three months in average). The results are globally similar to those in our study, i.e. a significant decrease in papular and pustular lesions between baseline and endpoint, in line with our findings. In the same manner, MTZ was shown to have efficacy on the erythema. On the contrary, its efficacy is absent on flushing and telangiectasiae. Tolerance was in general very good. QAE, as a newer therapeutic option, has only one trial published [9]. In this trial, its efficacy was demonstrated on all tested parameters (flushing, erythema, telangiectasiae, papules and pustules) over a six-week period. In this comparative study vs. MTZ 0.75% both products under study were shown to have a pretty similar efficacy on the reduction of papules and pustules counts, both starting to show significant results at D28. The same trend was followed on the improvement of erythema, whose reduction was significant with QAE as soon as D14, vs. D28 for MTZ.
Further, QAE showed a significant efficacy on the reduction of flushing from D14 onward, as MTZ had no effect on this parameter included at endpoint. As concerns telangiectasiae, QAE was shown to reduce them in a significant manner from D28 onward whilst MTZ was not showing any result till endpoint. These latter findings are in line with the reports published on MTZ. The global assessment was significantly improved with QAE as soon as D14, but only from D28 onward with MTZ. Tolerance was rated as very good for both products. The unique adverse event observed with MTZ was a severe allergic contact dermatitis, which was sometimes described with this topical drug [10,11]. The adverse event reported with QAE was allergic conjunctivitis whose occurrence did not appear to be related with the use of the product.
This significant and rapid efficacy of QAE may be explained by various properties of the triterpenoid quassinoids contained in this vegetal active ingredient. First, its antiparasitic activity which was previously reported on various parasites such as Plasmodium [12,13] and lice [6,7]. It was tempting to think that QAE could be effective on Demodex folliculorum (DF), whose role in the aetiology of rosacea is recognized. In another published study, we have demonstrated this antiparasitic activity of QAE on DF [14] by counting the number of mites extracted from biopsies of pustules of patients with erythematotelangiectatic and papulopustular subtypes of rosacea along a topical treatment with 4% QAE. These numbers reaching their physiological value after a 42 day course (mean n=0.9 at endpoint vs. 12.9 at baseline). There is no doubt that the strong antiparasitic activity of QAE on DF is a key factor in the efficacy of the same in the treatment of rosacea. Further, QAE was reported as having important antibacterial activity [15] by inhibiting, among others, the growth of Escherichia coli, Streptococcus faecalis, Stapylococcus aureus and Aspergillus niger. We have further demonstrated [14] its inhibitory activity in cultures of P. acnes and coagulase-positive Staphylococci where the growth of the bacteria was reduced in a significant manner (p<0.05) and at a lesser extent in cultures of coagulase-negative Staphylococci where the growth inhibition was not statistically significant. These antibacterial properties are important as increased expression of cathelicidin antimicrobial peptide (CAMP) was shown to be related to the pathogenesis of rosacea [16]. Staphylococcus epidermidis has been isolated predominantly from the pustules of rosacea patients but not from unaffected skin and may be transported around the face by Demodex mites [17]. Another experiment investigated is from 59 patients with diagnosed rosacea and a statistically significant correlation was demonstrated between positive reactions of the serum from these patients with B. oleronius antigens and the presence of Demodex mites on their eyelashes and facial skin lesions [18]. To the best of our knowledge, since the antibacterial activity of QAE was never demonstrated neither on Staphylococcus epidermidis nor on B. oleronius, its inhibiting activity was demonstrated on other species of Staphylococci [14,15]. This could be a promising way of investigation in the future. Third, QAEs were shown to potently inhibit the expression of tumor necrosis factor-alpha (TNF-alpha) and IL-1beta and IL-12 secretions in murine macrophages [19]. Skin sample from patients with rosacea analysis showed a higher expression of genes encoding pro-inflammatory cytokines (Il-8, Il-1b, TNF-alpha) leading to inflammation characteristic of rosacea [20]. About the mechanism of action of QAE on telangiectasiae, here is a suggested mechanism of action which should be confirmed.
It was reported that expression of VEGF receptors, both by vascular endothelium and infiltrating mononuclear cells, is observed in rosacea, contributing to the vascular changes and cellular infiltration that occurs in rosacea [20]. Mention was also made that histamine has an activity to induce VEGF production in the granulation tissue via the H (2) receptorcyclic AMP-protein kinase. A pathway and augments angiogenesis in the granulation tissue [21]. Among other neuroreceptors up-regulated in rosacea are the histamine receptors H2R [22]. On the other hand, it was demonstrated in an investigation about antiulcerogenic effects of QAE that these were probably acting via modulation of histamine H2 receptor [23]. This action could occur at skin level, and explain the effect of QAE on telangiectasiae. Summarizing, the remarkable effects of QAE on all the parameters of symptomatology of rosacea (flushing, telangiectasiae, erythema, papules and pustules) are due to a multifactorial activity of QAE.
We have demonstrated here in this randomized, double-blinded, parallel-group study that topical QAE had a more rapid and higher efficacy than MTZ on the treatment of the symptoms of rosacea. Whilst both drugs act similarly on papules and pustules, the efficacy of QAE is higher than MTZ’s one on the erythema but also on flushing and telangiectasiae, where MTZ has no activity. Of course, additional clinical trials involving larger groups of patients may be warranted to confirm these results..
Life Science Investments Ltd has provided the authors with free products (Quassia amara 4% cream) for this study.
CD has been a speaker for Life Science Investments Ltd and Isis Pharma.
AF has no conflict of interest.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Diehl C, Ferrari A (2017) Superiority of Quassia Amara 4% Cream over Metronidazole 0.75% Cream in the Treatment of Rosacea: A Randomized, Double-Blinded Trial. J Clin Cosmet Dermatol 1(3): doi http://dx.doi.org/10.16966/2576-2826.117
Copyright: © 2017 Diehl C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access