Introduction
Aortic dissection (AD) is the main cause of aortic intimal tear, which induces blood in the aorta to enter the aortic media, thereby rupturing the media and separating the two-chamber aorta [1]. AD is a relatively dangerous cardiovascular disease (CVD) with rapid progression and high mortality. At present, conservative and surgical treatments are mainly used in clinics. Thoracic endovascular aortic repair (TEVAR) has achieved a major paradigm shift in treatment of atherosclerotic thoracic, aneurysm, and chronic AD [2-3]. Traditionally, resting antihypertensive treatment has been the standard treatment for acute type-B AD.
TEVAR has become the preferred treatment for complex stanford type-B AD. However, differences are still observed on the treatment of non-complex stanford type-B AD. Many researchers advocate the use of TEVAR in treatment of acute AD, whereas some scholars believe that drug therapy should be adopted [4-5]. In this study, we investigated the long-term therapeutic effects of the two treatments and their role in suppressing the expression of inflammatory cytokines.
Methods
Patients
A retrospective analysis was conducted on 60 patients with acute AD admitted to the Cardiac Surgery Department of Xinjiang Uygur Autonomous Region People’s Hospital from April 2016 to January 2018. The patients were divided into control group (n=30) and TEVAR group (n=30) according to different treatment methods administered. Patients in both groups voluntarily participated and signed an informed consent.
Inclusion and exclusion criteria
The inclusion criteria are as follows: 1) patients who met the diagnostic criteria of acute AD [5], 2) patients with onset time to treatment time of less than 14 days, and 3) patients subjected to type-B dissection. The exclusion criteria are as follows: 1) patients with traumatic aortic pseudoaneurysm, aortic transection, and other injuries; 2) patients with onset time of more than 14 days of treatment; 3) patients with simple abdominal AD, connective tissue disease, and aortic penetrating ulcer; and 4) patients with systemic consumptive diseases, such as tumors and tuberculosis.
Therapeutic method
All patients were subjected to electrocardiogram (ECG) monitoring, sedation, and control of blood pressure and heart rate. Radial artery blood pressure was measured and pumped into Uradil (Heilongjiang Fuhe Huaxing Pharmaceutical Group Co., Ltd., Chinese medicine standard word H20040501, specification: 25 mg). If the effect of blood pressure reduction after 2 hours is not good, then felodipine sustainedrelease tablets are orally administered (Nanjing Yiheng Pharmaceutical Co., Ltd., Chinese medicine standard word H20103396). The systolic blood pressure was maintained at 100-120 mmHg, and diltiazem was pumped (Shandong Fangming Pharmaceutical Group Co., Ltd., national medicine standard word H20070254, specification: 10 mL: 10 mg) to control the heart rate at 60-70 times per minute.
TEVAR group: CT scanning was conducted to evaluate the condition of dissection, cerebral blood supply, anchorage area, and true or false lumen, determine the location of the entry, understand the type and specifications of the covered stent, and establish the appropriate surgical approach. After strict disinfection, the patients were placed in a horizontal position. A stent was placed in the target position after general anesthesia and tracheal intubation. After marking the position, angiography was performed again at the position of the stent to detect the presence of internal leakage and the artery and incision were sutured. The incision and blood circulation of the operative limb were carefully observed after the operation.
Indication of TEVAR therapy
Acute phase: 1. Dissection ruption and bleeding; 2. Progressive enlargement of peri-aortic or mediastinal hematoma; 3. Rapid enlargement of aortic diameter; 4. Severe ischemia of important branches of aorta; 5. Uncontrollable pain.
Chronic phase: 1. Dissection ruption and bleeding; 2. Dissection aorta diameter increased rapidly (>10 mm/year); 3. Formation of aneurysms (>50-60 mm); 4. Severe ischemia of important aortic branches (Malperfusion).
Sample collection
A total of 2 mL of fasting venous peripheral blood was collected 1 day before and 7 days after the treatment. Serum was collected after centrifugation (15 min, 15000 × g, 4°C), and stored at -80°C until use by following the protocols mentioned in previous works [6].
ELISA assays
Plasma levels of IL-6, IL-8, and TNF-α were measured by ELISA method before and after the treatment. The kits were purchased from R&D Systems, Minneapolis, MN. The numbers of white blood cells (WBC), neutrophils, lymphocytes, and monocytes were also measured by using Beckmann Kurt Uni Cel DxH 800 five-class hematology analyzer (purchased from Beckmann Kurt Commercial China Co., Ltd).
Evaluation of therapeutic efficacy
The evaluation criteria of curative effect included the following: the recovery of AD rupture is significant, the partial recovery of AD rupture is effective, and the failure of AD is ineffective. Total effective rate was calculated by (markedly effective+effective) cases/total cases × 100, and the in-hospital mortality rate is (in-hospital) cases/total cases × 100. The complications mainly include hemorrhage, renal insufficiency, infection, and cardiovascular events. The in-hospital incidence is evaluated by complications/total cases × 100%. In-hospital curative effect was compared with the total effective rate, in-hospital mortality, in-hospital time, and in-hospital complication rate of both groups.
Follow-up
Hospital survivors were clinically followed up with letters, emails, and phone calls and by the local referring physician when needed. Computed tomographic angiography (CTA) was performed before discharging all patients. After discharge, every patient was requested to repeat CTA at 1 month, 6 months, and annually after the primary TEVAR. Comparison of long-term and medium-term efficacy: 1-year and 2-year survival rates, incidence of complications after discharge, and secondary intervention rates were compared between the two groups. The complications after discharge mainly included internal leakage, distal recurrence of dissection, and progressive type-A layer. The incidence of complications after discharge is equal to the number of complications after discharge/the total number of complications × 100. The secondary intervention rate is equal to the number of secondary intervention cases/the total number of cases × 100. 1-year survival rate=1-year survival cases/total cases × 100%; 2-year survival rate=2-year survival cases/total cases × 100.
Statistical analysis
All data were analyzed using IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA). Data were expressed as mean ± SD. Categorical data were described as number and percentage. Student’s t-test was performed to measure the differences between the two groups. Fisher’s protected least significant differences test of ANOVA was also performed to analyze the quantitative data collected from both groups. Long-term survival was calculated using Kaplan– Meier method. A p value of <0.05 was used to denote significance.
Results
TEVAR suppressed the expression levels of inflammatory cytokines
The pre treatment analysis shows that there was no significant difference in the levels of IL-6, IL-8, and TNF-α in both groups (t=0.205, 0.194, 0.217, P>0.05). Post treatment result indicates that the levels of IL-6, IL-8 and TNF- α in both groups were significantly lower than those pre treatment (t=32.486, 22.451, 17.645, 45.612, 33.496, 47.813, P<0.01). The levels of IL-6, IL-8, and TNF-α in the TEVAR group were significantly lower than those in the control group (t=22.618, 18.746, 21.315, P<0.01). The levels of IL-6, IL-8, and TNF-α in TEVAR group post treatment was markedly lower than control group post treatment (t=36.362, 30.457, 33.652, P<0.01) (Figure 1).
TEVAR decreased the expression levels in immune cells
The pre treatment analysis of shows that there was no significant difference in the number of WBC, neutrophils, lymphocytes, and monocytes in both groups (t=0.187, 0.126, 0.249, 0.423, P>0.05). The post treatment result reveals that the number of WBC and neutrophils in both groups significantly decreased (t=5.784, 8.312, 9.015, 8.843, P<0.05), whereas the number of lymphocytes significantly increased (t=7.215, 9.142, P<0.05). The number of leukocytes and neutrophils in the TEVAR group was significantly lower than that in the control group (t=4.568, 3.216, P<0.05), and the number of lymphocytes in the TEVAR group was significantly higher than that in the control group (t=3.856, P<0.05). No significant difference in the number of monocytes between the two groups was observed in post treatment (P>0.05). Furthermore, there was no significant difference in the number of WBC, neutrophils, lymphocytes, and monocytes between TEVAR group post treatment and control group post treatment (t=0.128, 0.105, 0.172, 0.087, P>0.05) (Figure 2).
Comparison of the curative effect of both groups of patients
No significant difference in the basic characteristics of gender and age was observed in both groups (P>0.05) (Table 1). The post treatment result shows that the effective rate of the TEVAR group was significantly higher than that of the control group (P<0.05). No significant difference in the mortality, length of stay, and incidence of complications was observed in both groups (P>0.05) (Table 2).
| Parameter |
Control group (n=30) |
TEVAR group (n=30) |
χ2/t |
P |
| Gender (male/female) |
17/13 |
16/14 |
0.067 |
1.000 |
| Age (years) |
53.95 ± 5.65 |
51.89 ± 5.38 |
1.456 |
0.153 |
| Hypertension |
23 |
21 |
0.341 |
0.771 |
| Smoking |
12 |
13 |
0.069 |
1.000 |
| Diabetes mellites |
4 |
3 |
0.162 |
1.000 |
| Onset time (d) |
7.25 ± 0.81 |
7.41 ± 0.79 |
0.774 |
0.442 |
| Pseudo thrombosis |
|
|
1.017 |
1.000 |
| Thrombosis |
1 |
0 |
|
|
| False lumen patency |
29 |
30 |
|
|
Table 1: Basic characteristics of patients.
| Parameter |
Control group (n=30) |
TEVAR group (n=30) |
χ2/t |
P |
| Effective rate |
22 (73.33%) |
29 (96.67%) |
6.405 |
0.026 |
| In-hospital mortality rate |
3 (10.00%) |
2 (6.67%) |
0.218 |
1.000 |
| Length of hospital stay (d) |
23.12 ± 2.36 |
23.54 ± 2.41 |
0.682 |
0.498 |
| Complications in hospital |
4 (13.33%) |
0 |
4.286 |
0.112 |
Table 2: Comparison of curative effect of two groups of patients.
Comparison of mid-term and long-term efficacy of both groups
The 2-year survival rate and the incidence of complications after discharge of the TEVAR group was significantly higher and lower than that of the control group (P<0.05), respectively. No significant difference in the 1-year survival rate and the second intervention rate was observed in both groups (P> 0.05) (Table 3).
| Parameter |
Control group (n=30) |
TEVAR group (n=30) |
χ2/t |
P |
| 1-year survival rate |
25 (83.33%) |
27 (90.00%) |
0.417 |
0.748 |
| 2-year survival rate |
18 (60.00%) |
26 (86.67%) |
5.455 |
0.039 |
| Complications after discharge |
9 (30.00%) |
2 (6.67%) |
5.445 |
0.042 |
| Secondary intervention rate |
7 (23.33%) |
1 (3.33%) |
5.192 |
0.052 |
Table 3: Comparison of mid-term and long-term efficacy of patients.
Discussion and Conclusion
AD is one of the most dangerous forms of vascular disease, characterized by endometrial rupture and intramural hematoma formation. Generally, the pathological process is complicated and closely related to the infiltration of inflammatory cells into the aortic wall and apoptosis of vascular smooth muscle cells [7]. Inflammation is closely related to the prognosis of AD patients [8]. The suppression of inflammation could play significant role in reducing the incidence of complications in AD patients and improving the prognosis. The inflammatory cytokines (IL-6, IL-8 and TNF-α), released in the process of inflammation play important roles in the occurrence and development of CVD, such as myocardial infarction7. IL-6 can induce hepatocytes to synthesize high-sensitivity C-reactive protein and regulate macrophages to secrete TNF-α, which plays a key role in vascular injury and tissue trauma [9]. TNF-α is an early inflammatory factor released after AD. It can inhibit the proliferation of vascular cells, promote neutrophil phagocytosis, and damage vascular endothelial cells [10]. Previous studies reported that the plasma levels of IL-6 and TNF-α in AD patients were remarkably higher than those in healthy subjects [11,12]. Thus, monitoring the levels of IL-6 and TNF-α is helpful for clinicians to predict prognosis, and lower levels of IL-6 and TNF-α are beneficial to improve the prognosis in AD patients. The present study found that the levels of IL-6, IL-8, and TNF-α in both groups were significantly lower than those in the control group, whereas the levels of IL-6, IL-8, and TNF-α in the TEVAR group were significantly lower than those in the control group (Figure 1). One potential explanation for an occurrence of inflammation in AD is the involvement of systemic inflammatory response syndrome (SIRS), a clinical syndrome of dysregulated inflammation that includes a massive and uncontrolled release of pro-inflammatory mediators. The causes of SIRS are believed to be exogenous substances derived from pathogenic microorganisms, such as bacterial endotoxin, and endogenous substances that contribute to inflammation, such as those released by tissue damage. TEVAR seems to decrease the postoperative stress by offering less extensive incisions, dissection, and tissue manipulation. However, these beneficial effects may be offset by the release of cytokines during intra-luminal manipulation of the thrombus using catheters in endovascular repair, resulting in SIRS24. Therefore, our result suggests that TEVAR can significantly alleviate inflammation and improve the prognosis of patients.
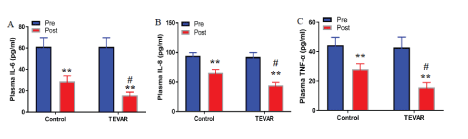
Figure 1: TEVAR suppressed expression levels of inflammatory cytokines.
ELISA was performed to detect the plasma concentrations of IL-6 (A), IL-8 (B), and TNF-α (C). Data are expressed as mean ± SD (n=30). Fisher’s protected least significant difference test was performed to analyze the differences between the control and TEVAR groups. **P<0.01 compared with that pre treatment; #P<0.01 compared with that post treatment.
The alternations of immune cells were closely related to the occurrence and development of AD [13]. Neutrophils have chemotaxis, phagocytosis, and bactericidal lamp functions that can release IL-6 and matrix metalloprotein-9 [14]. Del Porto, et al. [15] found that the total number of WBC and neutrophils in AD patients was significantly higher than that in healthy people. The studies have found that the mortality risk of AD patients in high WBC group was significantly higher than that in the low WBC group. Moreover, the ratio of neutrophil to lymphocyte is related to the prognosis of AD patients, and the ratio of neutrophil to lymphocyte in the death group is higher than that in the survival group [16,17]. In this study, we measured the levels of immune cells of patients before and after the treatment. The results showed that the number of WBC and neutrophils and the number of lymphocytes in both groups significantly decreased and increased, respectively. The number of WBC and neutrophils in the TEVAR group was significantly lower than that in the control group, and the number of lymphocytes was significantly higher than that in the control group (Figure 2). The destruction of the middle layer of the vascular wall delays the formation of early AD lesions and aortic aneurysms, subsequently reduces the number of WBC and neutrophils, and increases the number of lymphocytes [18].
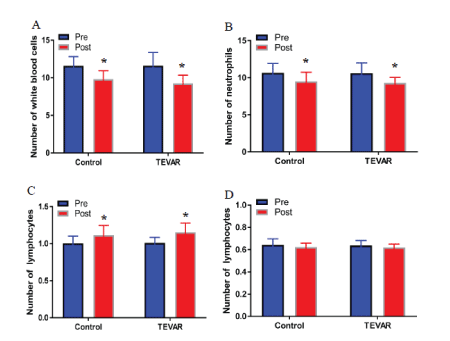
Figure 2: TEVAR decreased the expression in immune cells.
The number of WBC (A), neutrophils (B), lymphocytes (C), and monocytes (D) were measured by using a hematology analyzer. Data were expressed as mean ± SD (n=30). Fisher’s protected least significant difference test was performed to analyze the differences between the control and TEVAR groups. *P<0.05 compared with that pre treatment.
Previous studies reported that conservative treatment has adverse prognosis because approximately 30%-40% of AD patients can develop complex AD or aneurysmal degeneration [19]. The hospital mortality, complication rate, and secondary intervention rate in 30 days are high, but the 5-year survival rate is low [20]. The present study showed that the technical success rate of the TEVAR-treated group was high; its hospital mortality rate was low; the occurrence of severe complications, such as retrograde type-A dissection, and spinal cord ischemia, was low; and the short-term effect was satisfactory. Previous logistic regression analysis studies showed that age over 75 was an independent risk factor for early adverse events after surgery [21]. The results of this study showed that the effective rate of the TEVAR group was significantly higher than that of the control group. The mortality rate, length of stay, and incidence of complications in the TEVAR group were lower than those in the control group, but no statistical difference was observed in both groups (Table 2). Some studies also found that the 5-year, 10-year, and 14-year survival rates of AD patients after TEVAR treatment were 96.6%, 84.3%, and 67.8%, respectively, which were significantly higher than the long-term survival rate of the best drug conservative treatment [22]. Nienaber, et al. [23-25] found that the 5-year all-cause mortality of TEVAR group was 11.1% and 19.3%, and the aorta-related mortality was 6.9% and 19.3%, which were significantly lower than that of the drug treatment group. In this study, the 2-year survival rate and the incidence of complications after discharge of the TEVAR group was significantly higher and lower than those of the control group, respectively (Table 3).
In conclusion, TEVAR has good therapeutic effect that can greatly inhibit inflammation and improve survival rate to a certain extent. The small number of cases and no randomized controlled treatment are the limitations of present study. Therefore, a large sample of randomized controlled studies is still needed to investigate the therapeutic effect of TEVAR.
Acknowledgments
We thank Dr. Kyosuke Takeshita for carefully reading and applying changes to our manuscript.
Funding and Disclosure
This work, which was led by Zonggang Zhang and Lianjun Huang, was supported by the National Key R&D Program of China (Grant no. 2017YFC1308000). No duality of interest during the writing of this manuscript was declared by the authors.
Contribution Statement
MA, MY, WMZ, and ZZZ contributed to the conceptualization and formulation of the experimental design and the interpretation of the experimental results. MA, MY, WMZ, and AA performed the experiments and analyzed the data.MA, MY, and WMZ wrote the manuscript. MA, ZZZ, HJZ, and LJH ensured the integrity of the entire work. This manuscript was revised by its authors, all of whom decided to submit this version for publication.



