Abstract
A 60-year-old male who presented with cough and fever (case 1), and a 77-year-old female with hemoptysis (case 2) were admitted to IUHW Shioya Hospital. Interferon gamma release assay (IGRA: QFT-3G and T-SPOT.TB) were both performed on each patient; the QFT-3G was positive and T-SPOT.TB was negative in both cases despite different final diagnosis. While case 1 was determined to have active tuberculosis, in case 2 active tuberculosis was eliminated as the diagnosis. The contradictory results in these patients should serve as a reminder to clinicians that they should not rely on the results of IGRA testing for the diagnosis of active tuberculosis.
Keywords
Interferon gamma release assay; Negative; Tuberculosis; T-SPOT; QFT
Introduction
Interferon-gamma release assays (IGRAs) have been advocated for adjunctive tests for diagnose of active tuberculosis (TB) infection and they also have been advocated for alternatives to the tuberculin skin test (TST) for diagnose of latent TB infection (LTBI) [1-4]. Two types of IGRAs (QFT-3G and T-SPOT.TB) are currently available in Japan. The pooled sensitivities and specificities for the diagnosis of active TB by meta-analysis have been reported to be 0.80-0.81 and 0.79-0.99 for QFT-3G, and 0.81- 0.88 and 0.59-0.86 for T-SPOT.TB, respectively [2,3]. In low- and middleincome countries, where there is a high pre-test probability of TB, it was reported that neither TST nor IGRAs have value for predicting active TB diagnosis in adults, especially in the context of HIV coinfection [5]. In Japan, QFT-3G was initially approved for use in the public insurance system for the diagnosis of active or latent TB infection in 2009, and the T-SPOT.TB was subsequently approved in 2012. We report 2 patients with respiratory symptoms, both of whom had a positive QFT-3G and a negative T-SPOT.TB. One patient had active tuberculosis, the second did not. These contradictory results in an intermediate pre-test probability setting should be a cautionary warning in relying on the results of IGRA testing in the diagnosis of active tuberculosis [4,6].
Case Reports
Case 1
A 60-year-old Japanese male patient with a cough, decreased appetite, weight loss, and fever (>40°C) was admitted to International University of Health and Welfare (IUHW) Shioya Hospital in July, 2015. He had a past medical history of a cerebral infarction, hypertension, hyperlipidemia, and perianal abscess formation with a resulting diversion and stoma. The perianal abscess was not due to TB. His chest CT showed bilateral multiple inflammatory shadows with prominent bronchiectasis (Figure 1a). Blood tests revealed leukocytosis (24,000 cells/μl) with the following differential: neutrophils 92.8%, lymphocytes 2.4%, monocytes 4.7%, eosinophils 0% and basophils 0.1%. The HIV antibody test was negative, CRP was elevated (23.07 mg/dl). Results of IGRAs (QFT-3G and T-SPOT. TB) and TST were: positive, negative and positive, respectively (Table 1). Sputum yielded negative acid fast bacilli (AFB) staining for TB. Gastric aspirate yielded a positive AFB smear (gaffky 4) with a subsequent positive polymerase chain reaction (PCR) for TB. This patient was treated with a standard 4 drug TB regimen of isoniazid, rifampicin, ethambutol, and pyrazinamide with resolution of infection.
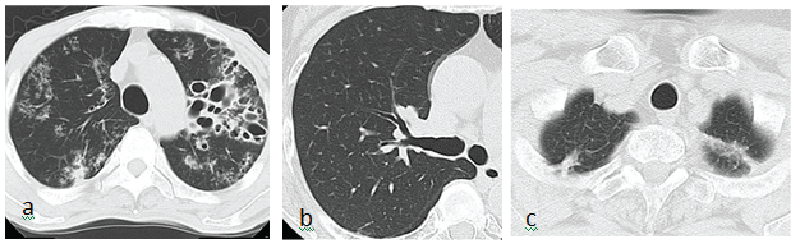
Figure 1: Chest CT. (a): Case 1. Prominent bronchiectasis mainly in the left lung and inflammatory shadows in the bilateral lung fields are seen. (b): Case 2. Bronchiectasis in the right upper lung field is seen. (c): Case 2. Inflammatory shadows in the bilateral upper lung fields are seen
Case 2
A 77-year-old Japanese female patient was admitted to IUHW Shioya Hospital because of hemoptysis (approximately 40 ml/d, for 3 days in March, 2016). She had been treated with anti-tubercular drugs for 6 months 15 years prior because of suspected pulmonary TB. Five days prior to the hemoptysis, the patient had developed respiratory tract symptoms including cough, sputum production, nasal discharge and a sore throat. Her chest CT showed bronchiectasis and old inflammatory shadows in bilateral upper lobes (Figures 1b and 1c). Blood test yielded elevated CRP levels (1.03 mg/dl) with normal white blood cell counts (5,040/μl) with decreased neutrophils population (neutrophils 20.2%, lymphocytes 62.5%, monocytes 9.4%, eosinophils 7.1%, basophils 0.7%). Bronchoscopy was performed and redness on the lumen of right upper lobe bronchus and bleeding from left B4+5 were observed. Bronchoscopy samples were negative for AFB staining, PCR for TB was negative. Gastric aspirate was also negative by AFB and PCR. Results of IGRAs (QFT-3G, T-SPOT.TB) and TST were: positive, negative and positive, respectively (Table 1). Additional bacterial and fungal evaluation yielded no significant pathogens. She was treated with antibiotics (garenoxacin 400 mg/d for 1 week and then levofloxacin 500 mg/d for 1 week) and hemoptysis resolved. She did not develop any further symptoms of tuberculosis and none of her mycobacterial cultures grew.
Case |
QFT-3G |
T-SPOT.TB |
TST |
1 |
Positive (2.62 IU/ml)
NC 0.40 IU/ml
PC 3.17 IU/ml |
Negative (0 spots, 2 spots)
NC 0 spots
PC 478 spots |
Positive
(0 × 0/ 20 × 12) |
2 |
Positive (2.43 IU/ml)
NC 0.22 IU/ml
PC>10 IU/ml |
Negative (1 spots, 3 spots)
NC 0 spots
PC 291 spots |
Positive
(0 × 0/ 12 × 12) |
Table 1: Results of interferon-gamma release assays (IGRAs, underlined) and tuberculin skin tests (TST). For the QFT-3G assay, blood was stimulated with a combination of three Mycobacterium tuberculosis specific antigens (ESAT-6, CFT-10, and TB7.7). For the T-SPOT.TB assay, the two results in the table were obtained from stimulation with ESAT-6 and CPT-10, respectively. For the positive control (PC) for both the IGRAs, stimulation was done using phytohemagglutinin. NC: negative control
Discussion
Although IGRAs have not been accepted as part of the criterion for diagnosis of active TB, it is common in Japan to use IGRA results to help in ruling in, or ruling out TB. These cases, which demonstrate contradictory results in one patient with and one without TB, highlight the pitfalls of over-reliance on the IGRA when diagnosing active TB. The hazard is over treatment for TB in the case of a false positive IGRA, or ruling out the diagnosis in a patient with a false negative test [7]. False negative tests are more likely in high pre-test probability setting (e.g., high prevalence countries), and false positives in low probability settings. Japan is currently a country with intermediate TB incidence (18 per 100,000 populations) [6].
There may be many explanations why these results may differ in the same patient. First is to understand the tests are methodologically different. The blood samples are stimulated with a combination of three Mycobacterium tuberculosis specific antigens (ESAT-6, CFT-10 and TB7.7) in QFT-3G. On the other hand, the separate stimulation with ESAT-6 or CFT-10 is used in T-SPOT.TB. While the total interferon-gamma concentration is measured in QFT-3G, the number of spots is counted in T-SPOT.TB. In addition, the effect of granulocytes agglutination detergent: T-Cell Xtend (Xtend) used in T-SPOT.TB might be important for the discrepant results. Second, the tests must be performed in a timely manner and delay or preservation may affect results. According to the T-SPOT.TB manufacturer’s instruction, the stimulation by ESAT-6 or CFT-10 should be started within 8 hours after the blood sampling from patients. If the reactions are started at 8 hours or later, the instruction guides to add Xtend to the samples. Xtend has been reported to allow the storage of whole blood for up to 33 hours without an impact on the results of the T-Spot.TB test [8]. When Xtend was added to the samples, the sensitivity of T-Spot.TB for active pulmonary TB was reported to be 0.70 (95% confidence interval [95% CI]=0.59-0.81) and was significantly (p<0.02) lower than QFT-3G (sensitivity 0.90; 95% CI=0.83- 0.97) in one report in Japan [9]. It is possible that the previously reported sensitivity for T-SPOT.TB was obtained from the data measured within 8 hours from blood sampling. For example, Kang et al. [10] reported the sensitivity of T-Spot.TB for active pulmonary TB as 0.92 (95% CI=0.83- 0.97) without using Xtend. The effects of Xtend have not been fully investigated or reported. Third, a positive IGRA may reflect past infection simply including LTBI and therefore may confound a current diagnosis since a substantial number of people will be positive at baseline regardless of current symptoms. Lastly there are data indicating that IGRA testing in persons with current TB disease may not be accurate, particularly under certain circumstances (e.g., age of ≥ 60 years, female sex and acute critical illnesses for QFT-3G, Malay for T-SPOT.TB, immunocompromised and Indian for both) [5,11,12]. These data did not explain why case 1, an active TB patient, not immunocompromised 60-year-old Japanese male, showed a false negative result of T-SPOT.TB only.
These two patients should remind physicians not to rely on the results of IGRAs for the diagnosis of active tuberculosis. Already established diagnosing methods (staining, culture, and PCR) cannot be replaced with IGRAs.
Conflict of Interest
The authors have no conflict of interest to declare.
Download Provisional PDF Here
Article Information
Article Type: Case Report
Citation: Umeda A, Yamane T, Inoue Y, Nishio K, Stauffer WM (2016) Contradictory Results between T-SPOT.TB and QFT-3G in Patients with Respiratory Symptoms. J Clin Case Stu 1(6): doi http://dx.doi.org/10.16966/2471-4925.133
Copyright: © 2016 Umeda A, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 04 Nov 2016
Accepted date: 07 Dec 2016
Published date: 13 Dec 2016


