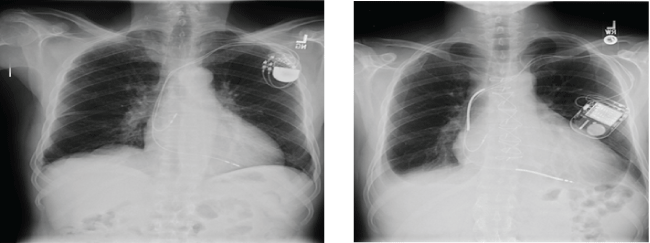
Figure 1: Pictures show the typical character of Chest X Ray of the patient with pacemaker (left) and with ICD (right). Please note the thicker radio-opaque coils in the right compared to those thin coils in the left.

Koichiro Nandate* Michael J Bishop
Department of Anesthesiology, Harborview Medical Center, University of Washington, USA*Corresponding author: Koichiro Nandate, Department of Anesthesiology, Harborview Medical Center, University of Washington, Box 359724 Ninth Avenue Seattle WA 98104-2499, USA, Tel: 206-744-7236; Fax: 206-744-8090; E-mail: knandate@uw.edu
Article Type: MINI REVIEW
Citation: Nandate K, Bishop MJ (2018) Emergency Perioperative Management of Cardiovascular Implantable Electronic Devices in Critical Trauma Patients. J Clin Anesth Manag 3(2): dx.doi.org/10.16966/2470-9956.139
Copyright: © 2018 Nandate K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Currently, approximately 3,000,000 people in North America have a cardiovascular implantable electronic device (CIED). Therefore, anesthesiologists have a frequent need to manage the patients with CIEDs. It is sometimes not straightforward, especially for critical trauma cases in which detailed information for either the CIED or the patient is unknown. We have created an algorithm for the practical perioperative management for the patient with a CIED who needs an urgent lifesaving surgical procedure, and for whom none of the information such as name, age, previous history of medical conditions or the type, manufacture and battery life of the device is available.
Cardiovascular implantable electronic devices; Critical trauma cases; Magnet
The number of patients with CIEDs has significantly increased in recent years, and it is estimated that at least 3 million people have a CIED in North America. It is also estimated that approximately 250, 000 new devices are implanted annually. Algorithms for the management of these devices in patients undergoing surgery have been published and endorsed by several national and international groups [1-3]. However, these guidelines or protocols are mostly oriented toward elective cases rather than for emergent, life-threatening cases. At a busy level 1 trauma center, we occasionally recognize that a patient has a CIED by the bulge on their chest wall, but we have no information about the type of device, the manufacturer, or when and why it was placed. It is very important for the anesthesia team to know if the device is a pacemaker (PM) or an implantable cardiac defibrillator (ICD), since the management of the patient differs depending on the type of device. The purpose of this manuscript is to introduce an algorithm for the practical perioperative management of critical trauma patients with CIEDs who need an emergent life saving surgical procedure and for whom little or no information is available as to the patient name, age, or previous history of medical conditions or the type, manufacturer, and battery life of the device.
Most of the previously published advisories or protocols have started with the importance of determining whether a device is a pacemaker (PM) or an implantable cardioverter defibrillator (ICD) by asking the patient, checking a wallet card, or looking up the patient’s medical records. However, in severe trauma, there may be little chance for the anesthesia team to collect information from either the patient or their records. Even in such a situation, it is possible for the anesthesia team to garner key information using the methods outlined in this manuscript.
Chest X-ray: Preoperative chest X-ray (CXR) can be useful to know whether a device is a PM or an ICD [4]. One of the most reliable ways to differentiate an ICD from a PM is to look for the presence of thicker shocking coils either in the superior vena cava or the right ventricle. Thicker shocking coils indicate the device is an ICD (Figure 1). Also, it should be noted that most current CIEDs have X-ray findings that can be used to identify the manufacturer of the device [5]. However, if the anesthesia team does not have enough experience to identify a device from the CXR or if the patient has bypassed the emergency department and has been brought directly to the operating room from the scene, CXR identification may not be possible.

Figure 1: Pictures show the typical character of Chest X Ray of the patient with pacemaker (left) and with ICD (right). Please note the thicker radio-opaque coils in the right compared to those thin coils in the left.
Magnet placement over the device: Magnet placement over the device to see the change in the electrocardiogram (ECG) rhythm is useful to help the anesthesia team to know if the device is a PM or an ICD. It is important to be sure that the monitor is set to “pacing on” to detect pacer spikes. There are five possible options for what will happen when a magnet is placed.
These options are summarized in (Figure 2).
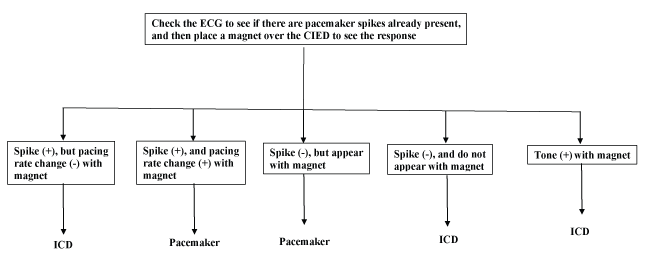
Figure 2: How to decide if the device is Pacemaker or ICD with use of magnet.
Regardless of the type of the device, the anesthesia team has to discuss with the surgical team the following case-specific information as to the possibility of electromagnetic interference (EMI) during surgery.
Use of electrocautery (monopolar or bipolar): The major source of EMI during surgery is generated from monopolar electrocautery. The common and potentially lethal effects of the electrocautery current are over sensing, initiation of the noise-reversion mode, and initiation of the electrical reset mode. Therefore, it is very important to know how much the surgical team plans to use monopolar electrocautery. Bipolar cautery is associated with no chance of generating EMI interference that will affect the device. However, in critical trauma surgery, monopolar electrocautery is almost mandatory to accomplish hemostasis as quickly as possible because it has multiple functions of cutting, dissection, and coagulation compared to the single coagulation function of bipolar electrocautery. Therefore, the generation of EMI by monopolar cautery is almost routine during trauma surgery.
Surgical sites (below or above the umbilicus): The anesthesia team must also know precisely where the surgical site is, especially whether it is below or above the umbilicus. If the surgical site is below the umbilicus, it is unlikely that EMI will be detected by the device. However, it should be noted that an inappropriate shock given by an ICD possibly due to incorrect positioning of the cautery dispersion pad that was not located on the ipsilateral but the contralateral side of the surgical site during knee surgery has been reported [6]. It may warn that the cautery dispersion pad can protect the device from EMI only when the cautery dispersion pad directs the current from the cautery site away from the device and leads. If the surgical site is above the umbilicus, the potential for EMI affecting the device becomes much greater. The management is different between a PM and an ICD, as the latter is more complicated. If the device is a PM, the necessity of reprogramming it to the asynchronous mode depends on the rhythm seen on the ECG. If the majority of the heartbeats are pacemaker generated, reprogramming to the asynchronous mode by magnet replacement may be required to prevent the inhibition of pacing by the EMI. If the rhythms are the patient’s intrinsic rhythm, no programming is needed. However, a magnet should be kept in the operating room because the rhythm might change to a paced one due to physiological changes in the patient caused by anesthesia and/or surgery. If the device is an ICD, it is more complicated. Magnet placement is required to prevent unnecessary shock, but is unable to convert the pacing function of the ICD to the asynchronous mode. Therefore, the inhibition of pacing by EMI cannot be prevented by magnet placement. The anesthesia team must monitor the pulse using an arterial line or plethysmograph. If the pulse becomes inadequate during cautery, the anesthesia team should inform the surgical team and ask them to at least minimize the duration and frequency of monopolar cautery. These are summarized in figure 3.
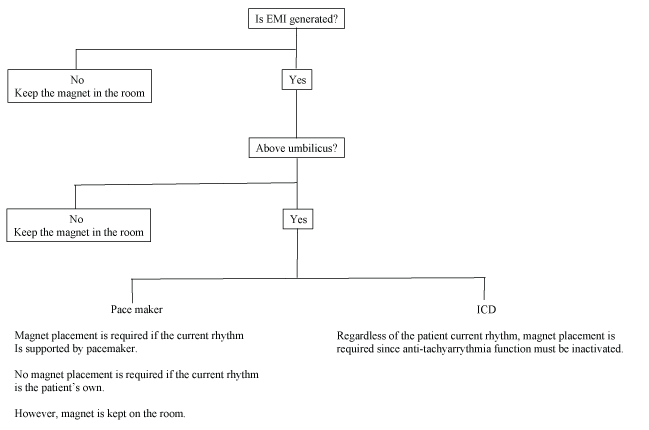
Figure 3: Algorithm of management of a CIED during critical trauma surgery.
Patient positioning: The proper placement of a magnet depends on the patient’s position. If the patient is supine, it is not difficult for the anesthesia team to place and keep the magnet in the proper position throughout surgery. The team can also administer any necessary external cardiac shocks manually and easily. However, in the case of either lateral or prone positioning, it is not easy to place and keep the magnet or to administer shocks manually. Fixing the position of the magnet with tape or an adhesive sheet may be a good idea. Also, putting the defibrillator pads on the patient prior to the surgery is safer.
The anesthesia team should appreciate that while EMI is a major consideration for CIED management, intraoperative anesthesia management may also affect CIED function. Most severe trauma patients are already intubated when they arrive in the OR, but some are not. If the anesthesia team administers general anesthesia and intubates with succinylcholine, they need to be aware of the possibility that the fasciculations induced with succinylcholine might inhibit the pacing since CIEDs may interpret the myopotentials of fasciculation as cardiac electrical activity [7]. Temperature management is also important. Shivering may inhibit pacing as a PM due to the myopotentials [8]. Hypothermia may affect the capture function because it may alter the pacing threshold of the myocardium, which could make the pacing output inadequate to initiate depolarization and may increase the arrhythmogenicity of the myocardium and may affect the function of a PM and/or an ICD [9]. The management of electrolyte and pH levels is also critical since they may induce capture failure because of the altered myopotential threshold. In severe trauma cases, most patients are resuscitated by massive blood transfusion, which induces disturbances of the electrolytes, pH, and temperature. Therefore, the anesthesia team has to consider the possibility of CIED malfunction induced by massive blood transfusion. All the factors that may affect the PM function are summarized in table 1. In addition to monitoring the ECG with “pacing on, “some monitoring of the perfusion is needed via either SpO2 or an invasive arterial line. This may be difficult for critical trauma cases due to severe vasoconstriction or hypoperfusion. A nasal alar probe is occasionally the best choice since the blood flow to the nose is supplied by a branch of the carotid artery, which has a preserved pulsatile waveform even when the limb pulses disappear.
| Factor | Effect | Mechanism |
| Shivering | Inhibit pacing function | Mis-sensed as cardiac function |
| Hypothermia | Affect capture function | Changes my potential threshold |
| Electrolytes changes | Affect capture function | Changes my potential threshold |
| Ph | Affect capture function | Changes my potential threshold |
Table 1: Factors which may affect the functions of CIED’s during critical trauma surgery.
In the immediate post-operative period in the post-anesthesia or intensive care unit, monitoring of the cardiac rate and rhythm as well as the peripheral pulse is required. Defibrillation and backup pacing device must be immediately available. Previous advisories and protocols have recommended the anesthesia team hand over the patient to a CIED team to examine the device as soon as possible to ensure it is working properly [1].
This manuscript describes an efficient and simple method of differentiating between a PM and an ICD using a magnet as well as how we can prevent lethal accidents induced by EMI during surgery on critical patients when information on the patient and their device is unknown. In such circumstances, our options are extremely limited, but we must proceed in order to save the patient’s life. Further, even in elective procedures, previously published guidelines mentioned that there were no absolutes regarding many clinical situations and our actions will vary in each individual case [1]. This is due to the wide variety of devices in terms of types, manufacturers, and functions. Communication between the teams and carefully monitoring the heart rhythm and peripheral pulse circulation are key. In this manuscript, use of a magnet has been recommended to differentiate the device type, a PM or an ICD, to reprogram a PM to the asynchronous mode and to suspend the tachyarrhythmia detection of an ICD. A chest X-ray sometimes helps but is not always available, especially in severe trauma cases. Recent published advisories do not recommend the intraoperative use of a magnet, but its use can be a key in emergencies. It should be noted that some devices can be programmed not to respond to magnet placement. Therefore, it is very important for the team to have a backup plan to support the cardiac rhythm and circulation with use of external pacing and/or a defibrillation device. In a previous advisory, “pacemaker dependency” was described as one of the decision-making factors for the clinical judgment of the necessity of the examination and reprogramming of CIEDs. However, in this manuscript, the usage of “pacemaker dependency” has been avoided. As mentioned, if the patient is being paced at the time of evaluation, it is almost impossible for the anesthesia team to determine what the rhythm would be in the absence of pacing. Therefore, the anesthesia team cannot determine if the patient is truly pacemaker dependent or not.
Download Provisional pdf here
All Sci Forschen Journals are Open Access