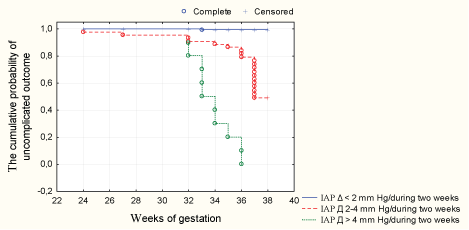
Figure 1: The functions of cumulative probability of uncomplicated pregnancy depending on three variants of the IAP elevation for 2 weeks of gestation.

Marshalov DV1* Shifman EM2 Salov IA1 Petrenko AP1 Ioscovich A3
1Department of Obstetrics and Gynecology, V.I. Razumovsky Saratov State Medical University, Saratov, Russia*Corresponding author: Dmitry Marshalov V, Razumovsky Saratov State Medical University, Department of Obstetrics and Gynecology of Medical Faculty, Saratov, Russia, E-mail: Marshald@mail.ru
Article Type: Research Article
Citation: Marshalov DV, Shifman EM, Salov IA, Petrenko AP, Ioscovich A (2017) Preeclampsia is a Syndrome of Intra-Abdominal Hypertension in Pregnancy - would a Hypothesis become a Theory?. J Clin Anesth Manag 2(1): doi http://dx.doi. org/10.16966/2470-9956.122
Copyright: © 2016 Ioscovich A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Recent studies offer new hypotheses of the development of preeclampsia (PE), which emphasizes the importance of intra-abdominal hypertension (IAH). IAH is an important factor in the alteration of intestinal perfusion) that can theoretically increase its permeability in pregnant women, cause bacterial and endotoxin translocation, systemic inflammation, and lead to PE symptoms developing; however, all existing hypotheses are based on empirical data and logical conclusions. This study was designed to corroborate a relation between the PE development, IAH and a violation of the barrier function of the intestine. Investigation of the dynamics of intra-abdominal pressure (IAP) was performed in 343 pregnant women in the period from 6 to 40 weeks of gestation. They were divided into 3 groups: Group I-215 patients with uncomplicated singleton pregnancy; Group II–patients with the risk factors of complications of pregnancy and childbirth outcomes without PE developing (n = 97); Group III - patients with PE (n=31). To determine the relationship between PE and affected intestinal barrier function, along with the study of IAP, starting from the second trimester of pregnancy, the intestinal permeability and the level of bacterial endotoxin were examined in parallel. We measured IAP indirectly by measuring the intravesical pressure, and calculated the compliance of the abdominal wall. The “lactulose/mannitol” test was used to assess the barrier function of the intestinal mucosa. Endotoxin levels were determined in serum by activated particles method (IRA - Endotox spp.).
IAP increases in linear fashion at a pace of approximately 1mmHg every two weeks throughout gestation The dynamics of the level of compliance of the abdominal wall had inversed linear relationship to IAP. Elevated IAP preceded the development of PE in 54.8% of cases. The rate of increase played a more significant role in the development of PE than the absolute value of IAP. The threshold for PE development was an increase in IAP more than 2-4 mm Hg for 2 weeks of gestation. Prognostic index of different values of the increase of IAP for the 2-week interval was 0.806. The study confirmed the presence of increased intestinal permeability in women with IAH, and its degree was dependent on the dynamics of IAP and correlated with the severity of developed PE (r=0.77). Increases in intestinal permeability were associated with the development of endotoxemia (r=0.82). IAH in pregnancy is one of the factors increased the intestinal permeability and endotoxemia. The translocation of bacteria and endotoxin triggers a systemic inflammatory response, multisystem violations. Monitoring of IAP dynamics in pregnant women provides new opportunities to predict PE.
Pregnancy; Intra-abdominal hypertension; Intestinal permeability; Preeclampsia
Recently, interesting publications linking the development of PE with IAH have appeared in the scientific medical literature. Existing theories of PE are reviewed in these studies and new pathogenic mechanisms are offered, the etiology of which, in one way or another, is associated with increased intra-abdominal pressure (IAP) during pregnancy [1-9]. New hypotheses are based on the results of the research by R.H. Paramore and his “mechanistic” conception of the development of PE proposed at the beginning of the last century [10-13]. In the 2000-s, there were discussions in some publications regarding possible association between IAH and PE, but the primacy of the IAH has been questioned [14,15]. The determination of causal relation between IAP and PE was hampered because the previous study authors investigated IAP only in the presence of the outcome being studied, not as a method of prediction.The first update of the mechanistic concept of PE development was conducted in 2011 by three authors independently and almost simultaneously [2-4]. Details of the concept had some differences, which complemented each other. The hypothesis was rooted in the identification of an imbalance of the reninangiotensin-aldosterone system [2-4]. Later this scenario of IAH related PE development was supported by other authors, which had confirmed hypotheses of A. van Dalfsen and H.J. Sugerman with new information [7- 9].The study demonstrated the importance of immunologic contribution to IAH development and the incidence of PE, which were associated with increased permeability of the intestinal wall, bacterial translocation and penetration of endotoxin (lipopolysaccharide - LPS), particles of microbial cells and their metabolic products into the internal environment. The same view was followed by other scientists. Developing their hypothesis, D.J. Sawchuck et al. [6] extended the understanding of the possible causes of PE, reinforcing information about the role of external and internal environmental factors: atmospheric pressure, living conditions, ecology and intestinal microbiome. In theory, the degree of translocation of LPS in pregnancy depends on two factors: the qualitative content of the intestinal microbiome and the degree of intestinal permeability. The intestinal microbiome is altered during pregnancy featured by an increase in titer of opportunistic gram-negative bacteria [16]. It has been shown in vitro that bacterial intestinal contents selected in the third trimester had significantly greater ability to activate the synthesis of pro-inflammatory cytokines compared with the microflora allocated in the first trimester of pregnancy [16]. In case of injury of the barrier function of the intestinal wall, LPS translocation occurs in the mesenteric lymph nodes and then moves through the portal vein to the liver [17,18]. LPS initiates a cytotoxic immune response in Kupffer cells with CD14 protein. Certain pro-inflammatory cytokines are genetically linked to the DNA of LPS that mediate the action of endotoxin in the systemic circulation and leads to a systemic inflammatory response, oxidative stress, accumulation of macrophage foam cell in capillaries, formation of atherosclerotic plaques and subsequent multiorgan failure [16]. The conventional opinion about the leading role of the placenta and fetus as a major factor of the immune response in PE is in doubt in the context of the above.IAH is an important factor for intestinal perfusion disorder that theoretically can increase intestinal permeability in pregnant women, leading to bacterial and LPS translocation, systemic inflammation, and development of PE symptoms. The study of relations between PE and IAP is impossible without evaluation of IAP preceding the arising complications. It is necessary to know not only the actual level of IAP, but also have an understanding of the normal values of this indicator in different periods of gestation. Until now, reference values of IAP during pregnancy were not available. Publications that contain factual information on the IAP during pregnancy and the postpartum period are rare and represent data of small samples [19-26].
To identify the relationship between the development of preeclampsia, intra-abdominal hypertension and intestinal barrier function impairment.
To achieve the objectives, the prospective, cohort study was done in period between 2008 and 2015. The study was approved by the Ethics Committee of V.I. Razumovsky Saratov State Medical University. Prerequisite was to receive an informed consent from patients to participate in the claimed study. Study of the dynamics of IAP was performed in 343 pregnant women in the period from 6 to 40 weeks of gestation. IAP was measured on an outpatient basis in the women who agreed to participate in the study when they were registering to the Women’s consultation (6-8 weeks) and in the ultrasound screening periods (20-24 and 30-34 weeks). IAP study in the hospital at different stages of gestation was conducted in pregnant women referred for examination due to asymptomatic bacteriuria, having risk factors for the development of obstetric and perinatal complications (women of older age groups and obese in varying degrees) as well as having diagnosed in-hospital gestational complications (fetoplacental insufficiency, fetal growth retardation, gestational hypertension and PE). In gestational terms of 38-40 weeks, IAP was studied in pregnant women, who were under supervision and patients with uncomplicated pregnancy, antenatal admission. Most of the pregnant women were surveyed repeatedly: twice at different stages of gestation - 127 patients, 3 times - 54. 4 times - 22. 140 patients -once before delivery.
Data of 215 pregnant women that met criteria were selected from total group to determine the reference values of IAP. The criteria for inclusion in the analysis were as follows: first, singleton uncomplicated pregnancy. Exclusion criteria included: presence of risk factors for IAH (obesity, intestinal dysfunction, postoperative scars on the anterior abdominal wall, adhesions process of abdominal, polyhydramnios, fetal macrosomia). We tracked the outcomes of pregnancy for all women included in our study to address the issue of pathogenic relations and primaсy between IAP and PE. PE was defined according to the classification of the International Research Society of Hypertension in Pregnancy (ISSHP) [27] as a gestational hypertension (systolic blood pressure>140 mm Hg or diastolic blood pressure >90 mm Hg identified in two or more control measurements after 20 weeks of gestation) with proteinuria (> 300 mg).Only results of reexamined patients were included in the study of dependence of pregnancy outcomes on the IAP, as it was necessary to analyze the data of I (6-8 weeks) and II (20-24 weeks) trimesters.Due to the multiple factors related to IAP, it was necessary to study the level of IAP, the period of reaching a critical value of IAP, and the duration of persistence of these measurements. Because of this, we studied that PE depends not only on the absolute values of IAP in particular term of gestation but on the rate of growth in IAP for certain time intervals, defined as Δ IAP. To determine the relationship between PE and injured intestinal barrier function, along with the study of IAP, we studied simultaneously intestinal permeability and the level of bacterial endotoxemia from the second trimester of pregnancy. We examined IAP using the indirect method of M.L. Cheatham et al.[28] with a closed system measuring an intravesical pressure UnometrTM AbdoPressureTM (Unomedical) consisting of a urimeter and measuring portion made of a graduated tube with steps of 1 mm Hg. In the supine position urinary bladder catheterization was performed by urinary Foley catheter connected to the connector UnometrTM Abdo-PressureTM, and then 20 ml of warm, sterile isotonic sodium chloride solution were injected into the bladder through the needleless port Kombi KonTM. Once the system was filled with a solution, the measuring part of the instrument was passed in a vertical position. We set the zero level of the scale at the symphysis and measured IAP.Latex two-way 8 Ch/Fr Apexmed catheter was used to minimize discomfort during catheterization. Sterile intraurethral gel Cathejell lidocain (Pharmazeutische Fabrik Montavit G.m.b.H., Salzbergstrasse 96 6060 Absam/Tirol Austria) was applied into urethra and on the catheter before the introduction for antimicrobial and local anesthetic action. The procedure was started 5 minutes after the onset of the therapeutic effect of the drug. During IAP examination we assessed the compliance of the abdominal wall by calculation according to the formula: the compliance of the abdominal wall = 100 / Δ IAP, where Δ IAP was the difference between IAP after administration of 100 ml of solution into the bladder and the initial IAP [29]. All ambulatory examined pregnant women were interviewed by phone for the presence of difficulty and pain when urinating for 2 weeks after examination. Test «lactulose / mannitol» test was used to assess the barrier function of the intestinal mucosa. This test was performed according to the method, proven for pregnant patients after an overnight fast and complete emptying of the bladder, patient drank 450 mL of a solution containing 40 g of sucrose, 7.5 g of 2 g of lactulose and mannitol during one hour from 8 a.m. to 9 a.m.. Sucrose provides pregnant with energy during fasting. Then the urine was collected in a sterile plastic container containing 10 ml of 10% thymol in isopropyl alcohol to prevent bacterial growth for 5 hours after administration of the prepared solution. Urine volume was measured and the samples were frozen at a temperature below 20°C until analysis. The analysis was performed using amperometric pulse detector by the method of ion chromatography. Results were expressed as the percentage of sugar in the urine samples. The ratio of lactulose/mannitol excretion was calculated by excretion percent [30]. The level of endotoxin was measured in the serum by the activated particles (IRA-Endotox spp), developed in Bakoulev Center for Cardiovascular Surgery (the committee’s decision on new medical technologies Health Ministry from 24.03.2004 g.) using standard kits. The sensitivity of the method was up to 7.5 pg / ml of lipopolysaccharide (LPS) of E. coli or Sal. typhi. The specificity of the method was 98.7-99.2%. STATISTICA software package was used for statistical processing (StatSoft Inc., USA, version 10.0). The results describing quantitative traits which empirical distribution did not show a statistically significant difference from the normal distribution, were presented in the form of (M ± σ), where M – the average value of sample, σ – standard deviation of the sample; if the difference between the normal law and empirical distribution was statistically significant, the results were presented as a median and an interquartile interval (Me [Q1; Q3]), where Me - median; Q1-1 (25%) quartile; Q3-3 (75%) quartile).
To assess the statistical significance of differences in mean values of quantitative indicators of the two groups, Student’s test was used for the options of equal and unequal variances.Test of the hypothesis about a statistically significant difference variance of the two groups was performed using Fisher’s test. Qualitative characteristics were described in percentage (%) and absolute values (n). To identify differences between groups by qualitative attributes, χ2 test with Yates correction was used due to low values of compared frequencies. The relationship between quantitative measures was assessed using Spearman’s rank correlation coefficient (r). Analysis of the relevant operating characteristics curves (ROC-analysis) was accepted to assess the overall prognostic potential, determination of prognostic indices of different values of the studied parameters, and determination of their threshold values with optimal sensitivity and specificity. To determine dependence of PE development on the rate of increase of IAP for certain time intervals, we calculated the cumulative probability of an uncomplicated pregnancy in patients with different rate increase of IAP during gestation using the methodology by Kaplan and Meier. Our calculation was based on the definition of conditional probabilities of uncomplicated pregnancy on each registered complicated outcome. For visual evaluation of the timing of PE development, we plotted the graph of function of a favorable outcome on the time (Kaplan-Meier curves) .In order to detect statistically significant differences between groups, Student’s t-test was used. Differences were considered significant if the value of p<0.05.
3 groups of patients were separated during the study. The patients with uncomplicated singleton pregnancy were assigned to the group I. As it was described previously, the results of these patients were used as a material for calculation of reference values of IAP at various stages of gestation. Pregnant women with risk factors for complications of pregnancy and childbirth outcomes without developed PE were assigned to the group II. Patients, whose pregnancy was complicated by PE, were assigned to the group III. Data presented in Table 1 show no significant differences in epidemiological characteristics of the patients of group II and group III. The rate of placental insufficiency and fetal growth retardation in patients with PE was specified basing on only those cases that were registered before the PE symptoms occurred. The study showed that the average IAP in patients with uncomplicated singleton pregnancy at 6 to 8 weeks of gestation were 1.40 ± 0.96 mm Hg; in the trimester II (20 to 24 weeks of gestation) –11.54 ± 3.40 mm Hg, and in the trimester III (27 to 41 weeks of gestation) –18.56 ± 1.35 mm Hg. Dynamics of the abdominal wall compliance had an inverse linear dependence towards IAP. The average values of the abdominal wall compliance were 43.20 ± 8.77 ml/mm Hg in the trimester I, 20.65 ± 5.10 ml/mm Hg in the trimester II, and in the trimester III the mean values of the abdominal wall compliance continued to decline to 13.03 ±1.30 ml/mm Hg. As shown in Table 2 representing the mean values of IAP in different stages of gestation, increases of IAP for a two-week interval in normal pregnancy did not exceed 1 mm Hg. The survey of patients in 2 weeks after ambulatory IAP measurement did not reveal any cases of catheterization related patient morbidity. The results of investigation of relationship between IAP and PE showed that the baseline (trimester I) values of IAP in the group with uncomplicated pregnancy were not significantly lower and not statistically significant compared with the group with PE. There were significant differences between groups in the second trimester. Meanwhile, the research of the strength of the relation between the level of IAP at the 20-24 weeks of pregnancy and the development of PE showed very weak correlation –r=0.023. p=0.752. The quality of analyzing the operating characteristics curves (ROC curves) for assessing the overall prognostic potential, determining prognostic indices of different values IAP and determining their threshold values was unsatisfactory –AUC=0.518. In the analysis of the results, it was noted that the reason for the lack of predictive value of the indicator “level of IAP” was significant difference between “normal” values of IAP in patients with different body mass index: patients with normal weight and uncomplicated gestation had average IAP of 10.83 ± 3.71 mm Hg; pregnant women with obesity I degree – 13.78 ± 1.68 mm Hg, with obesity II degree – 14.60 ± 1.52 mm Hg; with obesity III degree – 16.15 ± 1.14 mm Hg. The patients with PE complicated pregnancy had the following values of IAP at 20-24 weeks: patients with initially normal weight had IAB of 13.36 ± 3.93 mm Hg; with obesity I degree – 16.71 ± 0.76 mm Hg; with obesity II degree – 17.86 ± 1.96 mm Hg; with obesity III degree – 19.60 ± 2.06 mm Hg. IAP level preceding PE was significantly higher in pregnant women with obesity than in patients with normal weight (by 18- 32%, p<0.001). The reference values of IAP at 20-24 weeks of pregnancy in patients with obesity were: 13-15 mm Hg (obesity I degree), 14-15 mm Hg (obesity II degree), and 15-17 mm Hg (obesity III degree).
| Characteristics | I (n=215) n/% |
II (n=97) n/% |
III (n=31) n/% |
P-value |
| Average age (М ± σ) | 23.0 ± 3.1 | 28.3 ± 5.7 | 28.0 ± 3.9 | 0.946 |
| BMI (М ± σ) | 27.1 ± 2.7 | 35.1 ± 4.2 | 32.8 ± 5.7 | 0.554 |
| Obesity I degree | - | 23/23.7 | 9/29.0 | 0.634 |
| Obesity II degree | - | 16/16.5 | 5/16.1 | 1.000 |
| Obesity III degree | - | 10/10.3 | 4/12.9 | 0.742 |
| First pregnancy | 215/100 | 57/58.8 | 22/70.9 | 0.660 |
| First childbirth | 215/100 | 43/44.3 | 19/61.3 | 0.148 |
| Arterial hypertension | - | 64/65.9 | 20/64.5 | 0.999 |
| Diabetes | - | 2/2.1 | 1/3.2 | 0.568 |
| Diseases of kidneys | - | 10/10.3 | 3/9.6 | 1.000 |
| Fetoplacental insufficiency | - | 48/49.5 | 20/64.5 | 0.155 |
| Abnormal weight gain | - | 21/21.6 | 6/19.4 | 1.000 |
| Multifetal | - | 1/1.0 | 2/6.4 | 0.145 |
| Polyhydramnion | - | 1/1.0 | 2/6.4 | 0.145 |
| Fetal growth retardation | - | 10/10.3 | 7/22.6 | 0.124 |
| Fetal macrosomia | - | 2/2.1 | 3/9.6 | 0.091 |
| Early preeclampsia | - | - | 9/29.0 | |
| Late preeclampsia | - | - | 22/70.9 | |
| Moderate preeclampsia | - | - | 25/80.6 | |
| Severe preeclampsia | - | - | 6/19.4 |
Table 1: Epidemiological characteristics of patients in studied groups
Note: P-value - significance of differences between patients of group II and
group III.
| Gestation term | The number of pregnant | IAP (mmHg) |
| 6-8 weeks | 30 | 1 [0:3] |
| 20 weeks | 30 | 8 [7:10] |
| 24 weeks | 30 | 9 [8:11] |
| 27 weeks | 30 | 12 [11:13] |
| 32 weeks | 30 | 16 [15:16] |
| 33 weeks | 32 | 16 [16:16] |
| 34 weeks | 36 | 16 [16:17] |
| 35 weeks | 36 | 18 [17:18] |
| 36 weeks | 37 | 18 [18:19] |
| 37 weeks | 82 | 18 [18:19] |
| 38 weeks | 84 | 20 [19:20] |
| 39 weeks | 102 | 20 [20:21] |
| 40 weeks | 29 | 20 [20:21] |
Table 2: The average values of intra-abdominal pressure depending on
term of pregnancy (Me [QL; QU])
Note: Me-median; QL-25 percentile; QU-75 percentile
The study of relationship between IAP and PE showed that elevated levels of IAP preceded the development of PE in 54.8% of cases (n=17). In addition to IAP level, the rate of achieving the IAP critical values impacted the complications. Figure 1 showed the Kaplan-Meier curves for the three variants of IAP increase (Δ IAP < 2 mm Hg / 2 weeks of pregnancy, Δ IAP = 2-4 mmHg / 2 weeks of pregnancy, Δ IAP>4 mm Hg / 2 weeks of pregnancy), presenting the cumulative probability of uncomplicated pregnancy at a particular gestational term after 20 weeks.

Figure 1: The functions of cumulative probability of uncomplicated pregnancy depending on three variants of the IAP elevation for 2 weeks of gestation.
Results of subsequent multiple pairwise comparisons of functions of cumulative probability of uncomplicated pregnancy outcome related to PE showed that the patients with a higher Δ IAP had significantly lower probability of uncomplicated pregnancy than patients with IAP Δ<2 mm Hg. Timing of PE development at Δ IAP>4 mm Hg was significantly reduced–the median time of appearance of clinical signs of PE in these patients corresponded to 34.0 weeks of pregnancy (25% percentile–33.0 weeks; 75% percentile–36.0 weeks), while Δ IAP 2-4 mm Hg –37.0 weeks (25% percentile –36.0; 75% percentile –37.0). Assessing the overall prognostic potential, we performed determination of prognostic indices of different values of ΔIAP and determination of their threshold values by analyzing the operating characteristics curves (ROC curves) –AUC=0.806. Distribution of the points on the ROC curve appeared horizontal. The threshold value for PE was the IAP gain more than 2-4 mm Hg / 2 weeks of gestation. The study confirmed a direct relationship between IAP and intestinal permeability an increase in intestinal permeability due to increasing IAP: “lactulose / mannitol” ratio was 0.028 ± 0.001 at 20-24 weeks of gestation, when the level of IAP was 25-75 percentile, and it was 0.035 ± 0.001 (p <0.05) with IAP over 95 percentiles (greater than 12 mm Hg). Increasing IAH went along with increased ratio “lactulose / mannitol”: the Δ IAP 2-4 mm Hg / 2 weeks of gestation corresponded to the ratio 0.052 ± 0.003, and Δ IAP>4 mm Hg corresponded to the ratio 0.084 ± 0.002. In patients with moderate PE the ratio was 0.09 ± 0.002; in patients with severe PE the ratio was 0.158 ± 0.02 (p<0.001). The relation between “lactulose / mannitol” ratio and severity of PE was strong, correlation coefficient–0.77. The study demonstrated a relationship between the IAP and level of endotoxemia(r = 0.53, p <0.05). Women with IAP level corresponding to 25-75 percentiles showed a concentration of endotoxin of 7.2 ± 0.03 pg / ml, in pregnant women with IAP higher than the 95 percentile–9.4 ± 1 pg / ml (p <0.05). The correlation between the concentration of endotoxin in 20-24 weeks of gestation and the development of PE was below average, r=0.23, p=0.049. Endotoxin concentration was significantly increased with the progression of IAH: Δ IAP 2-4 mm Hg / 2 weeks of gestation corresponded to the concentration of 12.7 ± 2.0; Δ IAP>4 mm Hg corresponded to the concentration of 21.4 ± 3.1, and in patients the manifestation of severe PE–56.3 ± 4.2 (p<0.001). The coefficient of correlation between the ratio “lactulose / mannitol” and the endotoxemia level was 0.82.
Controversial expert opinions regarding PE pathogenesis and growing body of evidence of heterogeneity of the etiology of this complication of pregnancy confirm that PE is composite clinical category [32]. Recent studies show differences in clinical view, morphologic and immunohistochemical characteristics of the placental site, levels of biomarkers of placental and vascular damage in patients with different terms of the clinical presentation debut of PE [33-41]. We can assume the existence of several scenarios for the development of PE, one of which is the IAH [42].
The study showed that IAP in pregnant women IAP increases with gestational advancement at a rate of less than 1 mmHg per two week interval, it is less than 1 mm Hg in a two-week interval. Measurement of IAP according to the described method did not cause any urological complications. It was shown in the research of relationship between IAP and PE that elevated levels of IAP preceded the development of PE in 51.6% of cases. The rate of elevation of IAP played important role in PE development, even in a greater extent than the absolute value of IAP. Pregnancies with a rapid and substantial increase of IAP (4 mm Hg / 2 weeks of gestation) have significantly higher probability of PE development, than patients with IAP increase of less than 2 mm Hg for certain time interval (P<0.001), while the higher elevation of IAP the earlier the complication occurs. The threshold value for PE was IAP elevation more than 2-4 mm Hg / 2 weeks of gestation. Predictive index of different values of IAP elevation for the 2-week interval was 0.806. The results indicate the primacy of IAH in the cause-effect relationship in complicated pregnancy outcomes.The results of the study confirmed the presence of the development of IAP mediated increased intestinal permeability in pregnant women with IAH, and its degree depended on the dynamics of IAP and correlated with the severity of developed PE. Increased intestinal permeability was associated with a significant endotoxemia. The correlation between the concentration of endotoxin in 20-24 weeks of gestation and the development of PE was low –r=0.23, p=0.049. Several reasons may explain this phenomenon: one of variants involves penetration of a primarily hydrophobic form of endotoxin (without polysaccharide part) into the bloodstream and increased activity of antiendotoxin immunity that apparently occurs with a long-term and slowly progressive IAH in background in patients with obesity. The works of many researchers suggest that endotoxemia not only plays an important role in the development of PE, but the activity of antiendotoxic immunity [43-46]. Another reason for the low correlation of the initial concentration of endotoxin and PE is a rapid and significant increase of the concentration of the endotoxin with the progression of IAH, which was mainly observed in pregnant women with initially normal IAP. In its turn, this dependency can be explained by intake of hydrophilic forms of intestinal endotoxin, i.e. whole LPS molecule, with its polysaccharide part with increased intestinal permeability and rapid depletion antiendotoxic immunity on the background.
The quintessence of the work performed is the thesis that the intestine is the gateway to preeclampsia. Abdominal hypertension in pregnancy is one of the factors that increase the intestinal permeability leading to endotoxemia. The translocation of bacteria and whole LPS molecules triggers a systemic inflammatory response. Monitoring of IAP dynamics in pregnant women provides new opportunities of PE prediction.The results of our research present the first multi-factorial model of preeclampsia etiology, where the trigger mechanism is the abdominal hypertension syndrome. The material presented in this paper allows us to move from hypothesis to theory.
No Conflict of interest
Download Provisional pdf here
All Sci Forschen Journals are Open Access