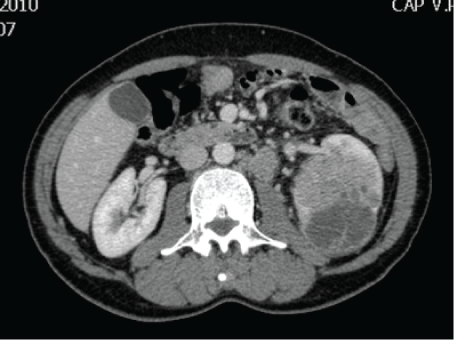
Figure 1: Abdominal CT-Scan showing a large left renal tumor measuring 8 × 8 × 7 cm with enlarged lymph nodes around the aorta.
Alshamsi H1 Al Muhrij AR2 Taha M3 Al Mousa R2*
1Imam Abdulrahman Alfaisal University, Dammam, Saudi Arabia*Corresponding author: Al Mousa RT, Urology Department, King Fahad Specialist Hospital, Dammam, Saudi Arabia, Tel: +966138442222; Fax: +966138371473; E-mail: riyad100@hotmail.com
Background: Renal cell carcinoma is not uncommon event in adults. Symptoms such as headache, confusion, altered behavior and seizures should raise the suspicion of brain metastasis. Most patients with brain metastases have concurrent metastases at other sites, thus, routine CNS (Central Nervous System) screening in asymptomatic patients without other common sites metastases is not recommended. Multidisciplinary approach is a must to treat such patients.
Case report: A 70-year-old gentleman with history of chromophobe renal cell carcinoma which was treated surgically and followed by adjuvant immunotherapy, presented 5 years later with history of headache, confusion, diplopia and weakness for 3 weeks’ duration. Upon investigations which included imaging and histopathological examinations, diagnosis of metastatic chromophobe renal cell carcinoma was established. Patient underwent craniotomy, excision of brain mass, radiotherapy and targeted therapy.
Discussion: Brain metastasis is not a rare event and the exact mechanism of late spread is still not fully understood. Isolated brain metastasis from renal cell carcinoma is a rare finding. Accordingly, brain imaging should not be part of routine surveillance after curative nephrectomy unless patient complained clinically. The best imaging modality is MRI and considered to be superior to CT scan due to its multiplanar capabilities and detection of small lesions. Histopathological examination confirms the diagnosis by revealing cell type. Brain metastases specific therapies include surgery, radiosurgery, conventional radiation, targeted therapies or combination of all mentioned methods.
Conclusions: Brain metastasis is not a rare event and should be considered in the differential diagnosis of patients who underwent nephrectomy for renal cell carcinoma as long as 10 years prior to presentation.
Chromophobe; Renal cell carcinoma; Brain metastases
Renal cell carcinoma is not uncommon in adults and represents 2-3% of all malignancies and about 85% of malignant renal tumors. The highest incidence is found in the sixth and seventh decades of life. It is subdivided into different histopathological types, where clear cell renal carcinoma is being the most common, followed by papillary and chromophobe renal cell carcinoma [1]. Chromophobe renal cell carcinoma usually metastasizes to the liver, lungs, lymph nodes and rarely to the brain [2]. Symptoms such as headache, confusion, altered behavior and seizures have been reported in 80–98% of patients with brain metastasis from chromophobe renal cell carcinoma [3]. Patients with brain metastases mostly have concurrent metastases at other sites upon diagnosis, thus routine CNS screening in asymptomatic patients without other common sites metastases is not recommended [4]. To our knowledge, there is no single recommended treatment or regimen for brain metastases of renal cell carcinoma. Hence, a multidisciplinary approach is recommended [3]. We are presenting to you a case report of a chromophobe renal cell carcinoma with brain metastasis with emphasizing the different aspects of diagnosis and management.
A 70-year-old gentleman was referred to our center with a history of gross hematuria and fever. He is a known case of benign prostate enlargement on tamsulosin 0.4 mg QHS. Clinical evaluation revealed a left renal mass. Abdominal and chest CT scan showed an 8 × 8 × 7 cm tumor in the left kidney with enlarged lymph nodes around the aorta (Figure 1). There were also minute lesions in both lungs, and bone scan showed no activities. He underwent left radical nephrectomy followed by adjuvant immunotherapy. The histopathological examination of the left kidney showed chromophobe renal cell carcinoma with metastasis to 3 lymph nodes out of 36, which was excised and the surgical margins were free (T3aN1M0).

Figure 1: Abdominal CT-Scan showing a large left renal tumor measuring 8 × 8 × 7 cm with enlarged lymph nodes around the aorta.
5 years later, he presented to the emergency department with history of headache, confusion, diplopia and weakness for 3 weeks’ duration. Brain CT scan was done and it showed a large 6.8 × 4.5 × 5.6 cm heterogeneously hyperdense mass with irregular border noted in the left frontal lobe. It was inseparable from the underlying bone and associated with bone destruction of the inner table of the left frontal bone. It had significant surrounding edema and mass effect resulting in midline shift to the right (1.5 cm from midline). Besides, there was a small osteolytic lesion with surrounding sclerosis within the left temporal bone above the mastoid air cells. Brain MRI showed left frontal bone density lesion with intracranial extra axial extension which showed intermediate signal intensity on T2 with heterogeneous signal intensity on T1 and multiple foci of high signal intensity consistent with foci of hemorrhage (Figure 2). Post contrast images showed heterogeneous, post contrast enhancement, and the remaining brain parenchyma showed normal grey/white matter differentiation. There were few scattered T2 and flair high signal intensities suggestive of the microangiopathic changes. CT brain angiogram showed the previously noted left frontal lesion with heterogeneous avid post IV contrast enhancement and similarly invasion of the adjacent bone with permeative pattern lytic area. There was an obvious feeding artery coming from the anterior branch of the left superficial temporal artery and possibly from middle meningeal artery draining into intracranial veins along the left superficial middle cerebral veins draining back into sphenoparietal dural venous sinus and cavernous sinus. Supply from anterior branches of left middle cerebral artery and left anterior cerebral artery cannot be ruled out.
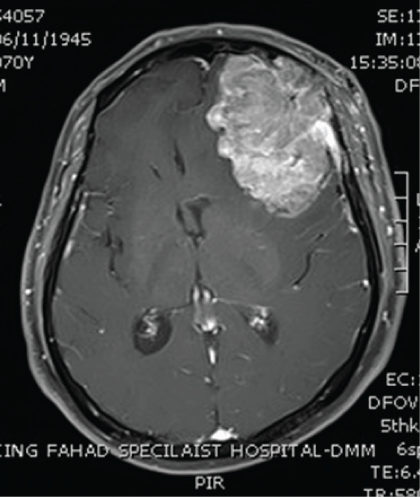
Figure 2: Brain CT-scan showing a large brain tumor involving the left frontal lobe and measuring 6.8 × 4.5 × 5.6 cm and also the surrounding edema and shift of the midline to the right.
Patient underwent craniotomy, excision of brain mass and radiotherapy. Histopathology of the excised brain lesion showed metastatic carcinoma involving the brain parenchyma and extending to the leptomeninges, dura and skull bone. The tumor is composed of solid sheets and large nests of malignant epithelial cells. The tumor is highly vascular, hemorrhagic and necrotic. Numerous hemosiderin laden macrophages are observed. The neoplastic cells are polygonal in shape and have abundant clear to eosinophilic/granular cytoplasm with enlarged nucleus and prominent nucleoli. As a result, diagnosis of metastatic renal cell carcinoma was established. The morphological features as well as the absence of cytokeratin 7 immunostaining, lack of colloidal iron, and focal positive immunoreactivity of the tumor cells with CD10, support the diagnosis of metastatic chromophobe renal cell carcinoma; eosinophilic variant.
Tumor Board decided to start him on targeted therapy in the form of PAZOPANIB 400 mg that was increased to 800 mg as patient tolerated the therapy. 9 months later, patient was found to have bone and lung metastasis that are unrespectable and recommendation was made to continue palliative targeted therapy. Patient is still alive after completing 16 months of targeted therapy with acceptable overall condition.
Brain metastasis is not a rare event and should be considered in the differential diagnosis of patients who underwent nephrectomy for renal cell carcinoma as long as 10 years prior to presentation [5]. The exact mechanism of late spread and brain metastasis is still not fully understood. However, a few theories have been suggested. These include: (1) Microscopic brain metastasis occurs early and it is a slow growing tumor and the symptoms will appear late (2) Microscopic brain metastasis occurs early and it grows rapidly whenever patient immunity decreases (3) Microscopic metastasis is formed in the lungs, and then degeneration and organization allow the metastasis to be carried to the brain as a micro embolism.
Isolated brain metastasis from renal cell carcinoma is a rare finding. Accordingly, brain imaging should not be part of routine surveillance after curative nephrectomy unless patient complained clinically [5]. The best imaging modality is MRI and considered to be superior to CT scan due to its multiplanar capabilities and detection of small lesions [6]. Most researches stressed that the size of the brain metastasis (not the number) is a major determinant factor for symptoms development and therefore the type of management that should be chosen [4].
Histopathological examination confirms the diagnosis by revealing cell type. Renal cell carcinoma has 5 subtypes: clear cell (75%), papillary (15%), chromophobe (5%), collecting duct (2%), and unclassified (3%) [1]. The incidence of chromophobe renal cell carcinoma metastasis is around 6-7% with the most common sites are liver, lung and lymph nodes. The importance of distinguishing chromophobe renal cell carcinoma from the clear cell type lies in the prognosis [2]. Most cases of chromophobe renal cell carcinoma are diagnosed at Stage I or II. Poor prognosis is often affected by the extracranial metastases. In such patients, a multidisciplinary approach is must. Brain metastases specific therapies include surgery, radiosurgery, conventional radiation, targeted therapies or combinations of all mentioned methods. Some factors are also important when considering the best therapeutic strategy. These include the performance status, number, size and location of brain metastases, the extension of systemic metastases and a well-controlled primary tumor. Recent literature review recommended using targeted therapies in combination and conjunction with whole brain radiation therapy and radiosurgery. Chemotherapy failure is usually attributed to the intact blood–brain barrier and acquired drug resistance by renal cancer cells [7]. To our knowledge IL-2 is the only approved agent known to produce long-term cure. IL-2 use in this patient population poses theoretic risks including peritumoral cerebral edema from capillary leak syndrome, cerebral hemorrhage from treatment induced thrombocytopenia, altered mental status, seizures, and treatment-related confusion. In renal cell carcinoma, the new agents sunitinib and sorafenib have demonstrated early promising results in treating CNS metastasis. A recent case report indicated that sunitinib was safe and led to a partial response in patients with brain metastasis of renal cell carcinoma [4].
Brain metastasis is not a rare event and should be considered in the differential diagnosis of patients who underwent nephrectomy for renal cell carcinoma as long as 10 years prior to presentation. CNS imaging should not be part of routine surveillance after curative nephrectomy, unless neurologic symptoms are present, evidence of metastasis at other sites such as lungs, or there is history of prolonged tobacco use. A multidisciplinary approach is important to treat such patients, including surgery, radiosurgery, conventional radiation and targeted therapies or a combination of all mentioned methods.
Download Provisional pdf here
Article Type: Case Report
Citation: Alshamsi H, Al Muhrij AR, Taha M, Al Mousa R (2017) Brain Metastasis from Chromophobe Renal Cell Carcinoma. Int J Cancer Res Mol Mech 3(2): doi http://dx.doi.org/10.16966/2381-3318.135
Copyright: © 2017 Alshamsi H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access