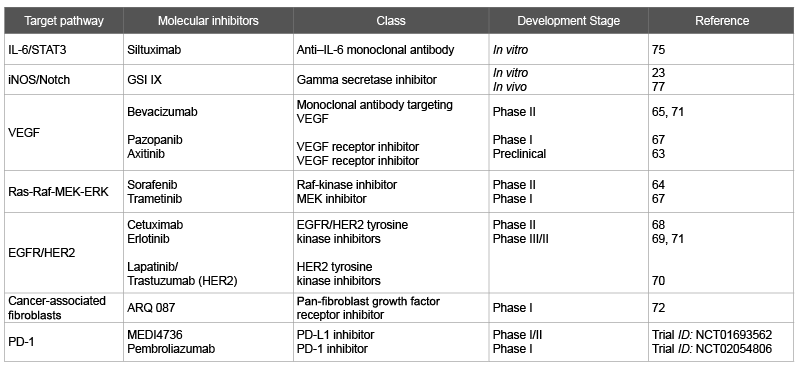
Table 1: Potential therapeutic molecular targets in cholangiocarcinoma in development
Elizabeth Park1 Jennifer Wu2*
1Department of Internal Medicine, NYU School of Medicine, USA*Corresponding author: Jennifer Wu, Division of Hematology and Oncology, Department of Medicine, NYU School of Medicine, Perlmutter Cancer Center, 550 First Ave, BCD 556, Bellevue Hospital, New York, NY 10016, USA, E-mail: jennifer.wu@nyumc.org
Aritcle Type: Review Article
Citation: Park E, Wu J (2015) Pathogenesis and Therapeutic Targets of Cholangiocarcinoma. Int J Cancer Res Mol Mech, Volume 1(2): doi http://dx.doi.org/10.16966/2381-3318.109
Copyright: © 2015 Park E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Cholangiocarcinoma is a potentially lethal cancer of biliary epithelium with variable incidence rates across the world. Given patients often have advanced disease at the time of diagnosis and limited therapeutic options, the prognosis of patients with cholangiocarcinoma remains poor. There are efforts to develop new treatments for cholangiocarcinoma as current standard chemotherapy offers limited benefit. In vitro and in vivo models of cholangiocarcinoma have been studied to unveil underlying molecular mechanisms of cholangiocarcinogenesis. Recent advances in understanding of different pathways underlying cholangiocarcinogenesis will guide the development of potential therapeutic molecular targets. We will do a detailed review of the pathogenesis of cholangiocarcinoma and its relevance in molecular targets for potential therapies in cholangiocarcinoma. Clinical trials using targeted therapies and proposals for potential clinical trials using novel targets will be discussed.
Cholangiocarcinoma is a rare but highly lethal cancer that arises from biliary epithelium, further classified based on location within the biliary tree as intra hepatic, perihilar and distal cholangiocarcinoma. This malignancy accounts for about 3% of all cancers with variable incidence in different countries [1]. The highest incidence is in Thailand (80-90 cases per 100,000 people) and the lowest incidence is in Australia (0.4 cases per 100,000 people) [2]. Studies have shown the increase in the incidence of intra hepatic cholangiocarcinoma in several countries, including the United States, Japan, Australia and England [3-6]. At Memorial Sloan-Kettering Cancer Center, the incidence of intra hepatic cholangiocarcinoma was compared to that of hilar cholangiocarcinoma from 1990 to 2006. Among 594 patients (intra hepatic = 270, hilar = 324), the average annual rate of growth for new intra hepatic cholangiocarcinoma was 14.2%, 3 times higher than that of hilar cholangiocarcinoma (P<0.001) [7].
As it is challenging to detect the disease at an early stage, cholangiocarcinoma is often diagnosed at an unresectable or metastatic stage and only systemic chemotherapy is considered standard of care. Standard first-line chemotherapy is gemcitabine plus cisplatin, based on a phase III, randomized controlled trial which showed significant median Overall Survival (OS) advantage in this combination group compared to the gemcitabine monotherapy group (11.7 vs. 8.1 months, respectively) [8]. However, among the same patient population, the median OS varies between 5 and 12 months, indicating the heterogeneity of cholangiocarcinoma [9-12]. Recent advances in molecular mechanisms underlying cholangiocarcinogenesis allow us to begin the understanding of the prognostic difference within this disease.
Overview: Many genetic mutations altering pathways that govern cell proliferation and survival have been discovered in cholangiocarcinoma [13]. Sia and colleagues performed genomic analysis on 119 tumor samples from patients with intra hepatic cholangiocarcinoma [14], and two distinct molecular classes emerged from this study: a proliferation class and an inflammatory class.
Inflammation: Chronic inflammation causes increased cell turnover, allows accumulation of mutations, and therefore plays an essential role in cholangiocarcinogenesis [2]. In addition, inflammatory mediators such as Interleukins (ILs) and Inducible Nitric Oxide Synthase (iNOS) are critical in various signaling cascades that regulate cellular proliferation and apoptosis in cholangiocarcinoma [15-18].
IL-6 mediates the expression of a growth factor called progranulin, which increases cholangiocarcinoma cell proliferation in vitro [15]. Progranulin is also upregulated in cholangiocarcinoma cell lines and patient tumor tissues [15]. IL-6 also induces phosphorylation of a transcription factor called signal transducer and activator of transcription 3 (STAT 3), which leads to increased resistance of cholangiocarcinoma cell lines to apoptosis by upregulating transcription of Myeloid Cell Leukemia-1 (Mcl-1), an antiapoptotic member of the B-cell leukemia-2 family, via an AKT-dependent pathway [16]. In a genomic analysis of 119 tumor samples from patients with intra hepatic cholangiocarcinoma, over expression of IL-6 was detected, in addition to constitutive activation of the oncogene STAT3 [14], further supporting the potential connection of a sustained IL-6/STAT3 signaling in cholangiocarcinogenesis [19].
INOS, a potent producer of nitric oxide, has been shown to induce Notch-1 expression in mouse cholangiocytes, which conferred resistance to apoptosis [17]. Moreover, upregulation of Notch-1 and iNOS expression was seen in cholangiocytes in patients with primary sclerosing cholangitis and cholangiocarcinoma, suggesting a link between the inflammatory mediator iNOS and Notch signaling. Indeed, several risk factors for cholangiocarcinoma such as primary sclerosing cholangitis [20,21], Opisthorchis viverrini, viral hepatitis [22-28] and intra hepatic stone disease [29,30], all of which expose cholangiocytes to a milieu of chronic inflammation that contribute to cholangiocarcinogenesis
Proliferation markers: Ras-MAPK-MET: In the study of Sia and colleagues, where 119 tumor samples from patients with intra hepatic cholangiocarcinoma were profiled, the proliferation class was associated with more aggressive tumors, characterized by poorer histologic differentiation and shorter survival compared to the inflammation class (median OS, 24.3 versus 47.2 months, respectively).
The patients who belong to the proliferation class had activation of oncogenic signaling pathways such as Ras-MAPK and MET, along with mutations in oncogenes KRAS and BRAF [14]. Ras-Mitogen-Activated Protein Kinase (MAPK) is one of the main signaling pathways governing cell growth and survival [31-33]. Ras is a guanosinetriphosphatase protein that activates various downstream pathways, including a signal transduction cascade comprising of activated protein kinases such as Raf, MEK, and MAPK, which play a key role in the intrinsic cell death pathway and transcription of pro-survival genes [32]. Growing evidence suggests that the mutations of oncogene KRAS may be involved in cholangiocarcinogenesis [33-36].
An analysis of tumor samples from 11 patients diagnosed with cholangiocarcinoma with history of primary sclerosing cholangitis indicates that KRAS mutation may have prognostic value [33]. In this group of 11 patients, KRAS mutation was observed in 4 patients. Patients whose tumors express KRAS mutation had shortened OS relative to that of patients with wild-type KRAS (5 ± 2 months versus 24 ± 7 months, respectively), suggesting the potential of poor prognosis association with of KRAS mutation in cholangiocarcinoma. Rashid and colleagues also supported the above data in a study evaluating genetic alterations and their association with clinic pathologic characteristics of the tumors in 33 Chinese patients with bile duct cancers [37]. KRAS mutation was present in 15% of patients, patients with KRAS mutation had worse prognosis compared to those without mutation (mean OS 3 ± 2.2 vs 15.5 ± 12.5 months, respectively). A study done by Australian investigators also showed similar incidence of K-ras mutations in patients with cholangiocarcinoma [38]. Of 60 patients with cholangiocarcinoma, KRAS mutation was detected in 8 (13.3%) patients. However, the study showed that neither Progression Free Survival (PFS) nor OS was affected by KRAS mutation status in these patients. Future studies using much larger patient cohorts would be helpful in determining prognostic value of KRAS mutation in patients with cholangiocarcinoma.
PI3K-AKT-mTOR pathway and HER pathway: Other signaling pathways involved in cholangiocarcinogenesis include Phosphatidylinositol 3-Kinase (PI3K)-AKT-mTOR and its mediator epidermal growth factor receptor (EGFR, also known as HER) family, which contains at least 4 subunits, HER1 (EGFR), HER2, HER3 and HER4 [39,40]. The PI3K-AKT pathway, which regulates cell survival and anti-apoptotic signals, is shown to interact closely with HER2 and EGFR in cholangiocarcinogenesis [39]. HER3 activates the PI3K-AKT pathway via p85 (adaptor subunit of PI3K) docking sites on the cytoplasmic tyrosine kinase domain [40]. Somatically acquired point mutations in EGFR gene were seen in the sequence coding for the tyrosine kinase domain in patients with biliary tree and gallbladder carcinoma [41]. In cholangiocarcinoma cell lines, exposure to EGFR kinase inhibitors led to prolonged EGFR activation and attenuated cell growth suggesting the therapeutic potential of tyrosine kinase inhibition in cholangiocarcinoma with activated EGFR [39].
In a study of 104 patients with cholangiocarcinoma, Andersen and colleagues analyzed transcriptional activity of the tumor samples, and demonstrated that poor OS and early recurrence was characterized by deregulation of oncogenic pathways including activated HER3 and EGFR signaling [42]. A recent retrospective study also supported the above finding, which showed about 30% of patients with localize or metastatic cholangiocarcinoma were positive for HER2 or HER3, and expression of HER2 was independent prognostic factor for mortality with hazard ratio of 3.08 [43]. Signaling pathways governing cell proliferation and survival that include PI3K and HER pathways have implications in cholangiocarcinogenesis and may help risk stratify patients by identifying those with worse prognosis.
PD-1 pathway: Cancer cells express tumor-specific antigens which can be targets of a tumor-specific T-cell response [44]. Specifically, these proteins expressed in cancer cells activate an anti tumor T-cell that mediates tumor killing, which can be inhibited by Programmed Death-1 (PD-1) pathway. PD-1 is a receptor expressed on T cells and tumorinfiltrating lymphocytes that exerts an inhibitory function on the immune system [45]. PD-1 has two ligands called Programmed Cell Death Ligand 1 (PD-L1, expressed on T cells, B cells and endothelial cells) and Programmed Cell Death Ligand 2 (PD-L2, expressed on macrophages and dendritic cells). Under normal physiologic conditions, when either PD-L1 or PD-L2 binds to PD-1, PD-1 pathway is activated and leads to inhibition of T-cell proliferation to prevent autoimmunity [45]. However, cancer cells can alter immune system to evade T-cell mediated death. one of the efficient strategies for tumor to survive is to upregulate PD-L1, which leads to increased PD-1 pathway activation. This results in subsequent cytotoxic T cell suppression to allow tumor to go undetected by the immune system [44]. These anti tumor T-cells are often found within infiltrating tumors, frequently called tumor infiltrating lymphocytes, and are known prognostic markers in many solid tumors including melanoma and breast cancers [46,47]. A recent study has shown that is also consistent in cholangiocarcinoma [48]. Cholangiocarcinoma tissue samples from 37 patients were analyzed with immunohistochemistry with markers including PD-L1 and CD45RO+. About 94% of sample was positive for PD-L1, raising its potential as therapeutic target. Patients whose tumor exhibited lymph node like structures (positive for CD45RO+) had better prognosis with better median PFS and median OS, suggestive of immune mediated suppression of tumor with tumor infiltrating lymphocytes. Tumor-infiltrating lymphocytes and expression of PD-L1 in cholangiocarcinoma can provide potential prognostic value and may have implication in the development of immunotherapy to treat cholangiocarcinoma.
Stromal factors: Cancer-associated fibroblasts, which make up most of stroma in cholangiocarcinoma, can contribute to tumor progression [2,49]. These fibroblasts are recruited and activated by cytokines released from cancer cells and inflammatory cells to make up stroma. Cancerassociated fibroblasts produce factors that influence the progression of cholangiocarcinoma via various mechanisms. Activated cancerassociated fibroblasts secrete cytokines such as VEGF, Fibroblast Growth Factor (FGF) and Hepatocyte Growth Factor (HGF). Such cytokines recruit macrophages, endothelial cells and inflammatory cells, which constitute tumor-promoting reactive stroma [50]. Hepatocyte Growth Factor (HGF) produced by cancer-associated fibroblasts in vitro has been shown to promote cholangiocarcinoma progression by enhancing cell motility and invasion [51]. In cell lines from patients with intra hepatic cholangiocarcinoma, cancer-associated fibroblast-derived periostin and its increased expression was associated with poor prognosis [52]. Cancerassociated fibroblasts also produce neurophilin-1, which helps tumor cell spreading by enhancing strength of the matrix supporting stroma [50,53]. Stromal factors can therefore be potentially utilized to predict prognosis in patients with cholangiocarcinoma.
Angiogenesis: Development of a rich vascular supply is required for cancer growth and spread. Angiogenesis is essential in cholangiocarcinogenesis, supported by increased expression of proangiogenic molecules such as Vascular Endothelial Growth Factor (VEGF) in cholangiocarcinoma cell lines and tissues [54,55]. VEGF promotes angiogenesis in tumor vasculature by inducing permeability and cell migration once it is bind to VEGF receptor [56]. Researchers studying cholangiocarcinoma xenograft models show that inhibition of VEGF expression is associated with decreased tumor cell proliferation and significant increase in apoptosis [57,58]. Strong association of angiogenesis and VEGF expression has been demonstrated in various solid tumors including lung and colorectal cancer, with success utilizing an inhibitor of VEGF in advanced disease with significant improvement of OS and PFS [59-64].
Targeting the IL-6/STAT3 pathway has already been proposed for the treatment of patients with metastatic renal cell cancer in a recent phase I/II study [65]. Siltuximab, an anti–IL-6 monoclonal antibody, showed signs of efficacy in metastatic renal cell carcinoma. Park and colleagues demonstrated growth inhibition of cholangiocarcinoma cell lines through IL-6 pathway blockade by either IL-6 neutralizing antibodies or MAPK inhiibitors [66]. Another potential therapeutic inhibitor studied in vitro or in vivo targets iNOS/Notch pathway [17,67]. When a γ-secretase inhibitor was applied, INOS/Notch could sensitize cholangiocarcinoma cells to TNF-related apoptosis-inducing ligand, a protein that induces apoptosis.
Ras-Raf-MEK-ERK pathway: Binimetinib, a selective small molecule inhibitor of MEK 1/2, was studied in a phase I clinical trial. This study enrolled patients with previously untreated advanced biliary cancer, and binimetinib was given in combination with gemcitabine and cisplatin [68]. Majority of them carried the diagnosis of cholangiocarcinoma; about 50% of patients achieved partial response and 30% had stable disease with the addition of binimetinib. An encouraging median OS of 9.1 months was reported and a phase II trial is in progress to evaluate safety and activity of binimetinib in biliary cancer.
HER Pathway: Despite compelling preclinical data suggesting potential use of EGFR (or HER) inhibitors in the treatment of cholangiocarcinoma, clinical trials have shown mixed results. In patients with advanced biliary cancer, the addition of cetuximab, a monoclonal antibody targeting the epidermal growth factor, did not enhance the activity of chemotherapy in a phase II trial [69]. The benefit of adding erlotinib to chemotherapy was studied in a randomized phase III clinical in Korean patients with metastatic biliary tract cancer (cholangiocarcinoma, gallbladder cancer, or ampullary cancer). Subgroup analyses showed that the addition of erlotinib to chemotherapy significantly prolonged median PFS in patients with cholangiocarcinoma (5.9 months for chemotherapy plus erlotinib versus 3 months for chemotherapy alone) without increase in grade 3 or 4 toxicities [70]. In a retrospective study, a subset of patients with advanced biliary cancer with HER2/neu amplification or mutation showed disease stability, partial response or complete response to HER2/neu-directed therapy (trastumumab, lapatinib or pertuzumab) [71].
Cancer associated fibroblast therapy: Therapeutic agents that interfere with cellular elements of cholangiocarcinoma stroma have been proposed [50]. In the 2015 ASCO annual meeting, Kyriakos and colleagues presented results of a phase I clinical trial studying ARQ 087, an oral Fibroblast Growth Factor Receptor (FGFR) inhibitor in patients with advanced solid tumors who failed standard therapy [72]. Among two patients with intra hepatic cholangiocarcinoma and FGFR2 fusions, one patient achieved a partial response and the second patient maintained stable disease with 26% decrease in target lesions, suggesting molecular pathway of FGFR could be a potential therapeutic target in cholangiocarcinoma.
Angiogenesis: Potential role of antiangiogenic therapy in cholangiocarcinoma is currently under investigation. A phase II clinical trial examined the benefit of the VEGF inhibitor bevacizumab to chemotherapy gemcitabine and capecitabine in patients with unresectable or metastatic cholangiocarcinoma. In such patients without prior systemic therapy for metastatic disease, PFS and OS were 8.1 and 11.3 months, respectively [73]. Another phase II clinical trial studied sorafenib, a multikinase inhibitor that targets both Raf and VEGF receptor tyrosine kinase signaling. It failed to demonstrate improved efficacy in advanced biliary cancer patients [74]. Oral administration of axitinib, a potent and selective second-generation VEGF receptor inhibitor, when added to gemcitabine, inhibited the growth of tumor in xenografts models, indicating its potential therapeutic use for cholangiocarcinoma [75].
EGFR inhibitor and anti-angiogenesis combination therapy: The combination of erlotinib and bevacizumab showed clinical activity in patients with advanced biliary cancer in a multicenter phase II trial [76]. Patients had either unresectable or metastatic disease at the time of diagnosis with no prior chemotherapy. About 12% and 51% of patients had partial response and stable disease, respectively. Median OS was 9.9 months, indicating a potential non-chemotherapy alternative in first-line treatment of cholangiocarcinoma.
MEK inhibitor and anti-angiogenesis combination therapy: Safety and efficacy of dual inhibition of MEK pathway and angiogenesis have been studied in patients with refractory cholangiocarcinoma in a phase I clinical trial [77]. Twenty-five patients with advanced cholangiocarcinoma who progressed through a median number of two prior therapies received pazopanib and trametinib in this trial. Median PFS and OS were 4.3 months and 6.7 months, respectively. Disease control rate, defined by partial response and stable disease, was about 75%. This combination therapy was well tolerated and showed modest activity in patients with highly refractory cholangiocarcinoma.
Immunotherapy: Adoptive cell therapy has also been proposed as a treatment of cholangiocarcinoma. Tran and colleagues have demonstrated that tumor infiltrating lymphocytes from a patient with metastatic cholangiocarcinoma had CD4+ T helper cells that recognized a mutation in HER2 interacting protein expressed by cholangiocarcinoma cells [78]. Patients who received adoptive transfer of tumor infiltrating lymphocytes had prolonged stabilization of disease, decrease in target lesions, and tumor regression [78].
While the above data show a potential utility of therapy targeting molecular pathway implicated in cholangiocarcinogenesis, there are no superior first-line treatment over a standard chemotherapy of gemcitabine and cisplatin in patients with unresectable or metastatic cholangiocarcinoma.
There have been great advances in revealing the underlying molecular mechanisms of cholangiocarcinogenesis. Tailoring pharmacotherapy based on specific signaling pathways implicated in cholangiocarcinogenesis may improve prognosis in patients with poor response to standard chemotherapy. As seen in Table 1 and Figure 1, there are many potential inhibitors that target specific signaling cascades are under investigation. Several of these compounds have shown efficacy in various malignancies but have limited data in patients with cholangiocarcinoma.
Development of in vivo disease model and randomized clinical trials inhibiting targets such as AKT or mTOR hold great promise in the treatment of cholangiocarcinoma. Immunotherapy such as PD-1 and PD-L1 inhibitors have potential therapeutic role in cholangiocarcinoma by removing inhibitory function of PD-1 on T cells to enhance immune killing of cancer cells. Many clinical trials on PD-1 or PD-L1 inhibitors have shown single agents activity in melanoma, renal cell carcinoma and non-small cell lung cancer [79]. Currently MEDI4736, a PD-L1- targeting antibody, is under investigation in phase I/II trials in patients with advanced solid tumors (NCT01693562). Phase I trial is in progress studying pembrolizumab, an anti-PD-1 antibody, in patients with advanced solid tumors (NCT02054806).
There is also a potential synergy in combining targeted therapies such as the inhibitors of inflammation (e.g., siltuximab) and the inhibitor of proliferation (e.g., trametinib) in cholangiocarcinoma. Combining immunotherapy and targeted therapies may also be a viable approach in the treatment of cholangiocarcinoma in the future.

Table 1: Potential therapeutic molecular targets in cholangiocarcinoma in development
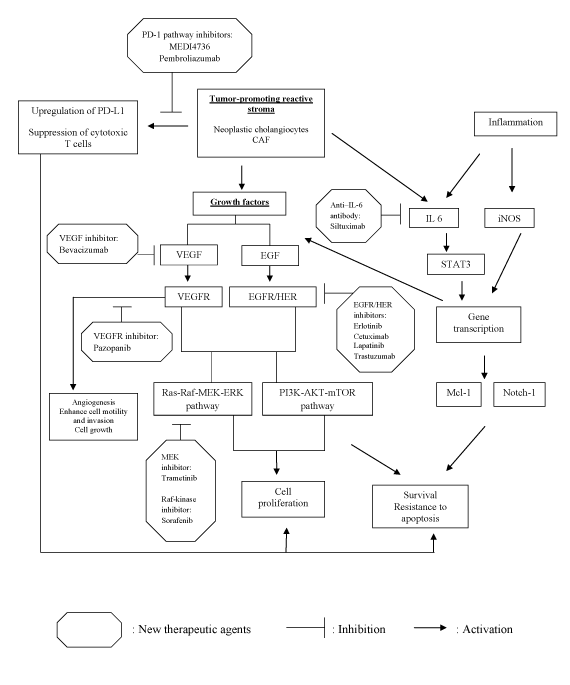
Figure 1: Multiple pathways contributing to cholangiocarcinogenesis and potential therapeutic inhibitors.
There is no conflict of interest for all authors.
Download Provisional pdf here
All Sci Forschen Journals are Open Access