Abstract
Non Hodgkin Lymphomas (NHL) complicating Hepatitis C virus (HCV) and Sjögren’s Syndrome (SS) share common characteristics. Genetic impairment of TNFAIP3/A20 contributes to lymphomagenesis in SS. We have studied genetic variants of TNFAIP3 in HCV patients with (n=87) and without (n=171) NHL. The prevalence of the rs2230926G variant did not differ between patients with and without NHL (p=0.51). However, among NHL patients, the rs2230926G allele was restricted to rheumatoid factor positive (RF+) patients (14.6% versus 0% in RF- patients, p=0.015). HCV infection is a new example of chronic antigenic stimulation where a coding variant of TNFAIP3 favor the lymphomatous escape of autoimmune B cells.
Keywords
A20; HCV-associated lymphomas; NF-kB
Introduction
Hepatitis C virus (HCV) infection is associated with an array of extra hepatic manifestations. Development of non-Hodgkin lymphoma (NHL) represents one of the most severe complications with an odd ratio (OR) ranging from 2 to 4 [1,2]. These lymphomas display frequent splenic or extra-nodal localizations. HCV infection is associated more specifically with marginal zone lymphoma (MZL) and diffuse large B-cell lymphoma (DLBCL), which frequently results from transformed MZL [3]. The presence of type II mixed cryoglobulinémie (MC), i.e. monoclonal immunoglobulin (Ig) IgM with rheumatoid factor (RF) activity, is a major risk factor of NHL development in HCV patients [4].
HCV infection is the prototype of chronic infection with a high level of blood circulating immune complexes due to long course of the symptom-free disease, high level of viral antigen (Ag) and high anti- HCV humoral response. IgG become immunogenic when they are present as immune complexes [5]. These immune complexes stimulate B cells bearing a membrane Ig with a RF activity i.e. secreting antibodies directed against the Fc portion of an IgG. Interestingly, De Re et al. showed that a large proportion of variable gene regions of HCV-associated NHL had sequences homologous with canonical RF sequences [6]. Of note, these restricted BCR gene segments found in HCV-associated NHL are very similar to those expressed by primary Sjögren Syndrome (pSS)-associated NHL [7]. Lymphomas complicating both pSS and chronic HCV infection share other remarkable characteristics, the predominance of MZL, the high frequency of mucosal localization, the association with MC and the localization of lymphomas in target organs where the chronic antigenic stimulation is active [8].
Our group has recently demonstrated that genetic variants of TNFAIP3/A20, which is involved in the control of NF-kB activation, promote the occurrence of MALT lymphoma in pSS patients [9]. This highlights that in the context of chronic antigenic stimulation, a dysfunction of a checkpoint of autoimmune B-cell activation could precipitate auto-immune B cells into a malignant transformation. Given the similarities between lymphomagenesis in pSS and HCV patients, we aimed to assess the role of genetic variants of A20 in HCV-associated lymphomas.
Methods
Study design and participants
Patients with HCV and lymphoma were selected from 2 cohorts. First, 69 patients were selected from the LymphoC study, an observational multicentric study that included adult patients with B-NHL and active HCV infection with the exclusion of those who were co-infected with HIV [10]. Cytological and histological samples were collected for centralized review and molecular analyses. This population was further supplemented by the addition of 18 patients previously genotyped in a GWAS performed in HCV and cryoglobulin-related vasculitis [11].
Control patients with HCV infection and no lymphoma were selected from 3 cohorts: 46 patients from the Department of Internal medicine of la Pitié-Salpêtrière hospital, Paris, 28 patients from the department of hepatology of Paul Brousse hospital, Villejuif, and 97 patients from the above mentioned GWAS performed in HCV and cryoglobulin-related vasculitis [11].
All the cases and controls were Caucasian. All patients gave their informed consent. Approval of the ethics committee from Paris Necker was obtained (n°05-06-08) for the Lympho-C study which was registered in clinicalTrials.gov (Identification number NCT01545544).
Blood samples and serologic analyses
Immunological analyses included serum Ig levels, and RF. Cryoglobulin determinations were carried out in each center. Positivity of MC was defined as a cryoglobulin level more than 0.05 g/L. A cytologic and phenotypic examination was performed in order to exclude the patients with more than 5% of circulating lymphoma cells, to focus on the study germline DNA.
Genotyping and whole exon sequencing
The rs2230926 single nucleotide polymorphism (SNP) which is located within exon 3 of the TNFAIP3 gene region was genotyped from germline DNA. Genotyping employed a predesigned TaqMan assay from Applied Biosystems (Foster City, CA, USA; assay no. 26882391-1) using a competitive allele-specific PCR system (LGC Genomics) as previously described [9]. Sequencing of the 9 exons TNFAIP3 gene was performed on germline DNA using the Sanger method as previously described [9]. Patients included in the GWAS mentioned above in the context of HCVassociated cryoglobulin have already been genotyped for the rs2230926 as previously described [11].
Statistical analysis
Continuous data were described as the median [range], categorical variables as number (%). Case-only associations (i.e., HCV patients with vs. without lymphoma) and comparisons between lymphoma histological types and immunological status were tested with Fischer’s exact test. A p-value <0.05 was considered significant.
Results and Discussion
Eighty-seven patients with HCV and NHL and available DNA were analyzed in this study. Histology subsets were 29 DLBCL, 37 MZL including 8 splenic MZL (SMZL), 5 mantle cell lymphomas, 8 follicular lymphomas, 2 chronic lymphoid leukemias and 1 chronic EBV-related lymphoproliferation. RF and MC were present in 48/87 (55.2%) and 43/66 (65.1) of the patients, respectively. These patients were compared to 171 HCV patients without NHL (Table 1). Among the controls, RF and MC were present in 127/171 (74.3%) and 118/143 (82.5%) of the patients, respectively. This high frequency of RF and MC in controls is due to the choice of the controls. As a first exploratory approach, 29 patients with HCV infection and lymphoma underwent TNFAIP3 whole exon sequencing of germline DNA. The missense exonic rs2230926G risk variant leading to an amino-acid replacement of Phe by Cys in exon 3 was present in 4 (13.8%). No other patient presented a TNFAIP3 variant in the coding exons. Thus, we decided to focus only on the rs2230926 variant for the other 58 patients with lymphomas and the 171 controls. Overall, there was no association between HCV- associated NHL and the rs2230926G variant found in 7/87 (8%) patients with NHL and in 19/171 (11.1%) patients without NHL (p=0.51) (Figure 1, upper panel). The same result was observed when comparing NHL patients to another large cohort of HCV patients without cryoglobulinemia (Laurent Abel; data not shown). Conversely to pSS patients, we did not find any association between the presence of this variant and histological subtypes; notably its frequency did not differ significantly between MZL and other subtypes (3/37 and 4/50 respectively, p=1). However, we found that, among NHL patients, the presence of the rs2230926G allele was restricted to RF+ patients (7/48 (14.6%) than in RF- patients (0/39 (0%), p=0.015) (Figure 1, lower panel right). Interestingly, there was no association between rs2230926G and RF in the control group with HCV infection but without NHL (rs2230926G in 13/127 (10.2%) RF+ patients compared to 6/44 (13.6%) RF- patients, p=0.58) (Figure 1, lower panel left), suggesting that the risk variant is not involved in RF activity emergence.
|
Non lymphoma patients
n= 171 |
Lymphoma patients n= 87 |
Demographic characteristics Male n (%) |
72 (42.1) |
39 (44.8) |
Immunology |
Age, years, median [(range]) |
57 [25-92] |
61 [24 - 87] |
Positive rheumatoid factor n (%) |
127 (74.3) |
48 (55.2) |
Lymphoma histology n (%) |
DLBCL |
NA |
29 (33.3) |
MZL |
NA |
8 (9.2) |
SMZL |
NA |
8 (9.2) |
FL |
NA |
8 (9.2) |
ML |
NA |
5 (5.7) |
CLL |
NA |
2 (2.3) |
PTLD |
NA |
1 (1.1) |
NS |
NA |
5(5.7) |
Table 1: Characteristics of HCV patients. DLBCL: Diffuse Large B Cell Lymphoma, HCV: hepatitis C virus; MZL : Marginal Zone Lymphoma, SMZL: splenic marginal zone lymphoma, FL: Follicular Lymphoma, ML: Mantle Lymphoma, CLL: Chronic Lymphoid Leukemia, PTLD: Post Transplant Lymphoproliferative Disease, NS: Not Specified
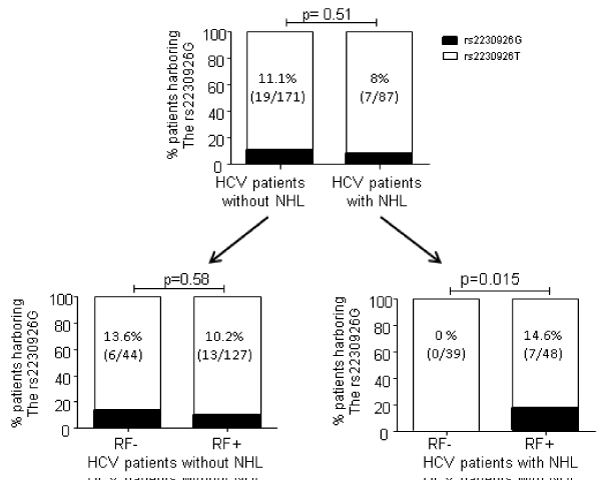
Figure 1: Frequency of the rs2230926G allele in HCV patients according to the NHL status (upper panel), the RF status in NHL (lower panel, right) or in NHL- (lower panel, left) patients. HCV: hepatitis C virus, NHL: Non Hodgkin Lymphoma, RF-: rheumatoid factor negative patients, RF+: rheumatoid factor positive patients
Altogether, we found an association between the missense exonic rs2230926 SNP of TNFAIP3 and the presence of RF in patients with HCVassociated lymphoma. The risk allele has been shown to be associated with a slight decreased capacity of control of the activation of the NF-kB pathway [9,12]. In contrast to our observation in pSS, this decrease of control of activation of NF-kB does not seem to induce lymphoma in the context of HCV infection since no association was found between NHL occurrence and exonic variants of A20. Conversely, our results highlight the possible involvement of A20 impairment in a multiple step process leading from chronic stimulation of RF+ B cells by immune complexes (IC) (anti HCVIgG/HCV-Ag) to NHL. RF-expressing clones are more prone than non-RF clones to transform into malignant lymphoma, due to chronic stimulation by abundant IgG-immune complexes [5]. Interestingly, it has been recently demonstrated that RF clones are rare in inflamed pSS salivary glands but are more prone to transform into malignant lymphoma [13]. To avoid lymphomatous escape of continuously stimulated RF+ B cells, a perfect control of the activation of the NF-kB pathway could be necessary and a slight dysfunction of this control may be sufficient for driving lymphomagenesis. This may explain why a germline variant of A20 may have specific consequences in RF+ B cells.
The reason why a germline polymorphism could have differential consequences in RF+ and RF- patients remains to be determined. One hypothesis is that the pathogenic process leading to lymphoproliferation in these two situations differs and that the pressure of microenvironment might not be the same. RF positive HCV-associated NHL, more frequently MZL, is dependent of a chronic stimulation by immune complexes containing HCV antigens. Increased NF-kB activation of these stimulated B cells could favor lymphoma escape. Conversely, RF negative HCV-associated NHL, more frequently DLBCL, could be induced by other mechanisms including directly infection of B cells by HCV which could promote the lymphoma transformation. In the latter cases, we could speculate that the SNP could protect from lymphoma development by increasing NF-kB activation in the infiltrating lymphocytes and/or macrophages and promoting a more efficient anti-tumor and/or antiviral response. Interestingly, it has recently been demonstrated that impairment of A20, as observed with the rs2230926G, in T CD8 infiltrating melanoma led to improve control of tumor growth [14].
In summary, this study extends our previous observation in pSSassociated MALT lymphoma to another example of lymphomas occurring in the context of chronic antigen stimulation. In both situations characterized by the chronic stimulation of RF+ B cells, a coding genetic variant of TNFAIP3 leading to a small functional defect of A20 function seems to favor the lymphomatous escape of these autoimmune B cells, but has no positive effect on lymphomagenesis from non-autoimmune B cells. Suppression of the chronic antigenic stimulation by effective treatment of HCV infection appears as a logical treatment of these HCV-associated lymphomas.
Conflicts of Interest
The authors have no conflict of interest to declare
Acknowledgements
We thank Laurent Abel (Institut Pasteur, Paris, France) who gave us access to genotyping data in a large cohort of HCV patients.
Funding and Grants
Discussion
The ANRS HC13 LymphoC study was funded by the sponsor ANRS (Agence Nationale de Recherches sur le Sida et les hepatites virales). Grant was obtained from NIH (R01DA013324 to D.T.).
Article Information
Article Type: Research Article
Citation: Nocturne G, Boudaoud S, Besson C, Davi F, Canioni D, et al. (2016) A Missense Variant of TNFAIP3 is Associated with the Presence of Rheumatoid Factor in Patients with HCV-Related Lymphoma. J Blood Disord Med 1(3): doi http:// dx.doi.org/10.16966/2471-5026.111
Copyright: © 2016 Nocturne G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 08 Oct 2016
Accepted date: 24 Oct 2016
Published date: 28 Oct 2016